ADME Toxicology Testing Market Size, Share & Trends Analysis Report By Product Type (Cell Culture Technology, High-Throughput Screening (HTS), Molecular Imaging Technology, OMICS Technology), By Method (Cellular Assay, Biochemical Assay, In-Silico, Ex-Vivo), By Application (Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Other Toxicities), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Contract Research Organizations (CROs), Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Adme Toxicology Testing Market Size
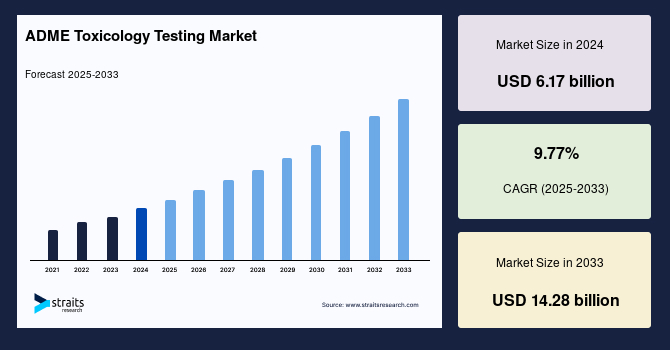
The global ADME toxicology testing market size was valued at USD 6.17 billion in 2024 and is projected to grow from USD 6.77 billion in 2025 to USD 14.28 billion by 2033, exhibiting a CAGR of 9.77% during the forecast period (2025-2033).
The ADME (Absorption, Distribution, Metabolism, and Excretion) Toxicology Testing Market encompasses a range of in vitro, in silico, and high-throughput methodologies to evaluate drug candidates' pharmacokinetic and toxicological profiles. These assessments are pivotal in early-stage drug development, ensuring that compounds are effective and safe before advancing to clinical trials.
The market's growth is propelled by the escalating demand for novel therapeutics, particularly in addressing chronic diseases such as cancer, diabetes, and cardiovascular ailments. For instance, in 2024, Insilico Medicine leveraged its AI-driven platform to present five preclinical cancer drug programs at the AACR Annual Meeting, showcasing the integration of advanced technologies in drug discovery. Furthermore, opportunities within the market are amplified by technological advancements, including the adoption of organ-on-chip models and AI-driven predictive analytics. Companies like Emulate, Inc. have introduced innovations such as the Chip-R1, designed to enhance the accuracy of in vitro models by minimizing drug absorption, thereby improving biological modeling.
Adme Toxicology Testing Market Trends
Integration of Artificial Intelligence (ai) in Adme Testing
The adoption of AI and machine learning in ADME toxicology testing is transforming drug discovery by increasing speed, accuracy, and predictive capacity. AI-powered platforms can process complex datasets from high-throughput experiments to model drug absorption, distribution, metabolism, and excretion pathways in silico.
- For instance, in December 2024, Charles River Laboratories, in partnership with Valo Health, expanded their AI-based drug development platform “Logica” to include integrated ADME and toxicity modeling, streamlining early-stage drug assessments. Similarly, Schrödinger Inc. unveiled new AI tools for predictive toxicology, enabling real-time forecasting of off-target effects.
These advancements reduce late-stage drug failures, shorten development timelines, and cut costs by highlighting promising candidates early. The predictive capabilities of AI are increasingly being used to design safer drugs with optimized ADME profiles from the outset.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 6.17 Billion |
| Estimated 2025 Value | USD 6.77 Billion |
| Projected 2033 Value | USD 14.28 Billion |
| CAGR (2025-2033) | 9.77% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Promega Corporation, PerkinElmer Inc., Agilent Technologies, Corning Inc., Bio-Rad Laboratories |
to learn more about this report Download Free Sample Report
Adme Toxicology Testing Market Growth Factors
Rising Demand for Novel Therapeutics
The global burden of chronic and infectious diseases intensifies, leading to demand for safer, faster-to-market therapeutics. According to the World Health Organization (WHO), over 20 million new cancer cases and a rapid rise in metabolic and neurological disorders were reported in 2022–2023, fueling the urgency for innovative treatments. Pharmaceutical companies are thus prioritizing preclinical safety evaluations through robust ADME testing.
- For example, in November 2024, AstraZeneca increased its investment in in vitro ADME labs to support pipeline expansion in oncology and rare diseases. Likewise, Novartis launched a dedicated early development program focusing on precision medicine, incorporating high-throughput ADME assays for compound screening.
Such trends emphasize how ADME toxicology testing is central to reducing attrition rates and boosting the success of clinical drug candidates.
Market Restraining Factors
High Costs Are Associated with Advanced Testing Technologies
Despite the transformative potential of advanced ADME toxicology testing technologies, their adoption is significantly constrained by high costs. Cutting-edge in vitro systems such as organ-on-chip, 3D bioprinting, and microfluidic-based assays require substantial capital investments in laboratory infrastructure, sophisticated equipment, and specialized personnel. Moreover, integrating AI-driven predictive models adds further expenses related to software licensing, data management, and computational resources. These factors can be especially burdensome for small and medium-sized enterprises (SMEs) and academic research labs operating under tight financial constraints.
As a result, many promising innovations in ADME testing remain underutilized or inaccessible. This financial barrier not only delays the democratization of high-precision toxicology assessments but also slows the broader pace of pharmaceutical innovation, limiting the ability of emerging drug developers to optimize safety and efficacy profiles early in the pipeline.
Market Opportunities
Expansion into Emerging Markets
Emerging markets such as India, Brazil, and Southeast Asia are becoming hotbeds for pharmaceutical innovation due to rising healthcare expenditure, government support, and growing clinical trial activities.
- For instance, in January 2025, Eurofins Scientific expanded its Asia-Pacific footprint by acquiring a cutting-edge ADME lab in Singapore, strengthening regional testing capabilities. Likewise, Labcorp Drug Development announced the acquisition of a predictive modeling startup in South Korea in 2024 to accelerate service delivery in East Asia.
These initiatives open doors to affordable, tailored solutions for drug developers in emerging economies, significantly broadening market reach and accessibility. Moreover, collaborations between global pharmaceutical companies and local research institutions in emerging markets can lead to the development of cost-effective and region-specific ADME testing solutions, further driving market growth.
Regional Insights
North America: Dominant Region
North America holds the largest share in the global ADME toxicology testing market, driven by advanced healthcare infrastructure, significant investment in pharmaceutical research, and stringent regulatory standards. The presence of major pharmaceutical companies and research institutions fosters a robust ecosystem for drug development. The U.S. Food and Drug Administration (FDA) enforces rigorous guidelines for drug safety evaluation, necessitating comprehensive ADME toxicology testing. Technological advancements, such as adopting high-throughput screening and artificial intelligence, have further propelled the market.
- The U.S. ADME toxicology testing market is driven by its robust pharmaceutical ecosystem, cutting-edge research institutions, and a strong regulatory framework. The FDA mandates comprehensive ADME profiling as part of Investigational New Drug (IND) applications, making such testing integral to early-stage drug development. Additionally, high investment in precision medicine and biotechnology fuels innovation in predictive toxicology tools.
- Canada’s ADME toxicology testing market is driven by strong government support for life sciences research, growing biotech startups, and increasing preclinical trial activities. Health Canada's emphasis on non-animal testing methods and the country's collaborative ecosystem between academia and pharma companies foster innovation in predictive toxicology. Additionally, partnerships with U.S.-based companies and funding incentives for early-stage drug development encourage the adoption of high-throughput and automation-based ADME testing technologies across Canada’s expanding pharmaceutical sector.
Europe: Fastest-Growing Market
Europe represents a significant ADME toxicology testing market characterized by a strong pharmaceutical research base and an innovative healthcare ecosystem. The European Medicines Agency (EMA) provides comprehensive guidelines for drug safety assessment, fostering a structured framework for toxicology testing. The region's commitment to reducing animal testing has increased investment in alternative methods, such as in vitro and silico approaches. Collaborative research initiatives between academic institutions and pharmaceutical companies have spurred innovation in testing methodologies. Countries like Germany, the U.K., France, and Italy are at the forefront, contributing significantly to market growth.
- Germany’s ADME toxicology testing market stems from its world-class pharmaceutical and life sciences industries. The country’s strong academic–industrial partnerships promote pharmacokinetics and drug safety innovation. Moreover, Germany’s adherence to EU regulations and emphasis on early-stage in vitro and in silico ADME testing enhances the development of safer drugs and supports market expansion.
- The UK’s ADME toxicology testing market benefits from its strong biopharma infrastructure, robust academic research base, and regulatory alignment with EMA standards. Government initiatives such as the UK Life Sciences Vision (2021) and funding from Innovate UK support drug safety innovations and alternative testing models. The increasing focus on reducing animal testing and adopting AI-driven in-silico models is shaping demand.
Asia-Pacific: Rapid Growth
Asia-Pacific is witnessing rapid ADME toxicology testing market growth due to rising pharmaceutical R&D investments, expanding CRO presence, and supportive government initiatives in countries like China, India, and South Korea. The region’s large patient population and rising clinical trial activity drive demand. Additionally, cost-effective testing services and increasing adoption of in vitro methods to reduce animal testing boost market expansion. Regulatory reforms to align with international standards are also enhancing market growth and innovation across Asia-Pacific.
- China's ADME toxicology testing market is witnessing rapid growth due to its expanding pharmaceutical sector and reforms to align with international drug approval standards. The National Medical Products Administration (NMPA) now mandates detailed ADME data for its regulatory review. Furthermore, increased investment in drug R&D and the rise of domestic biotech firms are amplifying demand for high-throughput ADME screening.
- India’s ADME toxicology testing market thrives because of its cost-effective manufacturing capabilities and expanding clinical trial landscape. Regulatory bodies like the ICMR and CDSCO encourage pharmacokinetic studies to ensure drug safety and efficacy. India’s emergence as a global hub for generic drugs and biosimilars has led to an uptick in preclinical testing, including ADME profiling. Additionally, collaborations between local CROs and global pharma companies fuel technological upgrades and market growth.
Analyst Opinion
As per our analyst, the global ADME toxicology testing market is set to witness robust expansion in the coming years, fueled by growing demand for safer, faster, and cost-effective drug development processes. As drug candidates become more structurally complex and therapeutic areas expand into oncology, neurology, and personalized medicine, the importance of early pharmacokinetic and toxicological assessment has intensified. High-throughput screening platforms, AI-driven predictive modeling, and organ-on-chip technologies are transforming how ADME profiling is conducted, making it more accurate and scalable.
Despite these innovations, the market still faces several challenges. High initial costs associated with advanced testing platforms and a global shortage of highly trained personnel in pharmacokinetics remain notable restraints. Furthermore, small and mid-sized biotech firms often struggle with the affordability of such technologies, which can limit widespread adoption.
To sustain momentum, strategic collaborations between academic institutions, contract research organizations (CROs), and biopharma companies are critical. In parallel, government initiatives supporting translational research and drug discovery, especially in emerging economies, will play a key role in accelerating market adoption and ensuring long-term growth.
Segmentation Analysis
By Product Type
High-Throughput Screening (HTS) has emerged as a pivotal segment in the market. HTS enables rapid evaluation of vast compound libraries, efficiently identifying potential drug candidates. The increasing demand for efficient and automated screening methods has propelled the growth of this segment. For instance, Eurofins expanded its ADME/Tox testing capacity in the Asia-Pacific region by constructing a new bioanalytical laboratory in Singapore, emphasizing the growing importance of HTS in drug discovery and development.
By Reinsurance Type
In-silico methods, involving computational modeling and simulations, have gained traction due to their cost-effectiveness and ability to predict ADME properties without extensive laboratory experiments. These methods accelerate drug development by identifying potential failures early, thus saving time and resources. Integrating artificial intelligence and machine learning has further enhanced the predictive capabilities of in-silico models, making them indispensable in modern toxicology testing.
By Application
Systemic Toxicity stands out as the leading application segment in the market for ADME toxicology testing. This type of testing is essential for evaluating the overall adverse effects of a drug on the entire body, not limited to a single organ system. It plays a pivotal role in early-stage drug development to identify compounds with harmful systemic effects. As pharmaceutical companies strive to ensure comprehensive safety profiles, systemic toxicity testing remains a cornerstone for regulatory compliance and successful clinical progression.
By End-User
Pharmaceutical and biotechnology companies represent the largest end-user segment in the market. The increasing investment in drug development, expanding pipelines, and a focus on personalized medicine have amplified the demand for comprehensive ADME toxicology testing services. These companies prioritize early-stage testing to identify potential failures, ensuring the safety and efficacy of drug candidates before clinical trials.
List of Key and Emerging Players in ADME Toxicology Testing Market
Key players like Thermo Fisher Scientific, Charles River Laboratories, and Bio-Rad Laboratories dominate through expansive service portfolios and robust R&D. Strategic acquisitions and AI-driven platforms enhance predictive testing accuracy. Increasing demand for in vitro and in silico testing methods is prompting firms to invest in automation and cloud-based data analytics. Collaborative projects with pharma and biotech companies are boosting global market presence, especially in regulatory-compliant toxicology services across North America, Europe, and Asia-Pacific.
- Promega Corporation
- PerkinElmer Inc.
- Agilent Technologies
- Corning Inc.
- Bio-Rad Laboratories
- Covance Inc.
- Charles River Laboratories
- Eurofins Scientific
- Thermo Fisher Scientific
- GE Healthcare
- Cyprotex
- Evotec AG

to learn more about this report Download Market Share
Recent Developments
- January 2025- Eurofins Scientific expanded its ADME testing capabilities by acquiring a state-of-the-art laboratory in Singapore. This strategic move aims to strengthen Eurofins' presence in Asia-Pacific, catering to the growing demand for advanced drug metabolism and pharmacokinetics studies. The acquisition is part of Eurofins' broader strategy to enhance its global laboratory network and service offerings.
- In 2024, India's National Institute of Pharmaceutical Education and Research (NIPER) signed a public-private partnership with a major U.S. biotech company to build ADME testing hubs with AI and bioinformatics tools.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 6.17 Billion |
| Market Size in 2025 | USD 6.77 Billion |
| Market Size in 2033 | USD 14.28 Billion |
| CAGR | 9.77% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Method, By Application, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
ADME Toxicology Testing Market Segments
By Product Type
- Cell Culture Technology
- High-Throughput Screening (HTS)
- Molecular Imaging Technology
- OMICS Technology
By Method
- Cellular Assay
- Biochemical Assay
- In-Silico
- Ex-Vivo
By Application
- Systemic Toxicity
- Renal Toxicity
- Hepatotoxicity
- Neurotoxicity
- Other Toxicities
By End-User
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Contract Research Organizations (CROs)
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































