Antibody Specificity Testing Market Size, Share & Trends Analysis Report By Product & Services (Products, Antibody Validation & Specificity Testing Services), By Technology (Genetic Validation-based Technologies, Microarray-based Antibody Technologies, Immunoassay-based Technologies, Western Blotting, Immunochemistry, Flow Cytometry, Others), By Application (Research & Development, Clinical Diagnostics), By End Use (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Diagnostic Laboratories, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Antibody Specificity Testing Market Overview
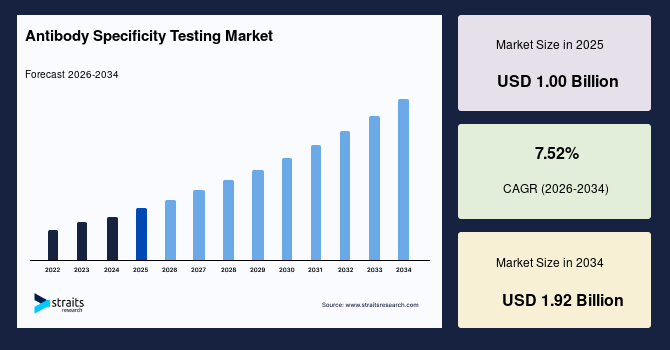
The global antibody specificity testing market size is valued at USD 1.00 billion in 2025 and is estimated to reach USD 1.92 billion by 2034, growing at a CAGR of 7.52% from 2026-2034. The exponential growth of the global market is driven by rising demand for ultra-sensitive cross-reactivity analytics in personalized biologics pipelines, uniquely driving antibody specificity testing expansion globally.
Key Market Trends & Insights
- North America dominated the global market, accounting for 39.83% share in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 9.18%.
- Based on products & services, the products segment accounted for the largest market share in 2025.
- By technology, the genetic validation-based technologies segment is estimated to register the fastest CAGR growth of 8.72% during the forecast period.
- By application, the clinical diagnostics segment is projected to grow at a CAGR of 8.65% during 2026-2034.
- By end use, the pharmaceutical & biotechnology companies segment dominated the market with a revenue share of 53.06%in 2025.
- The U.S. dominates the market, valued at USD 342.53 million in 2024 and reaching USD 366.82 million in 2025.
Table: U.S. Antibody Specificity Testing Market Size (USD Million)
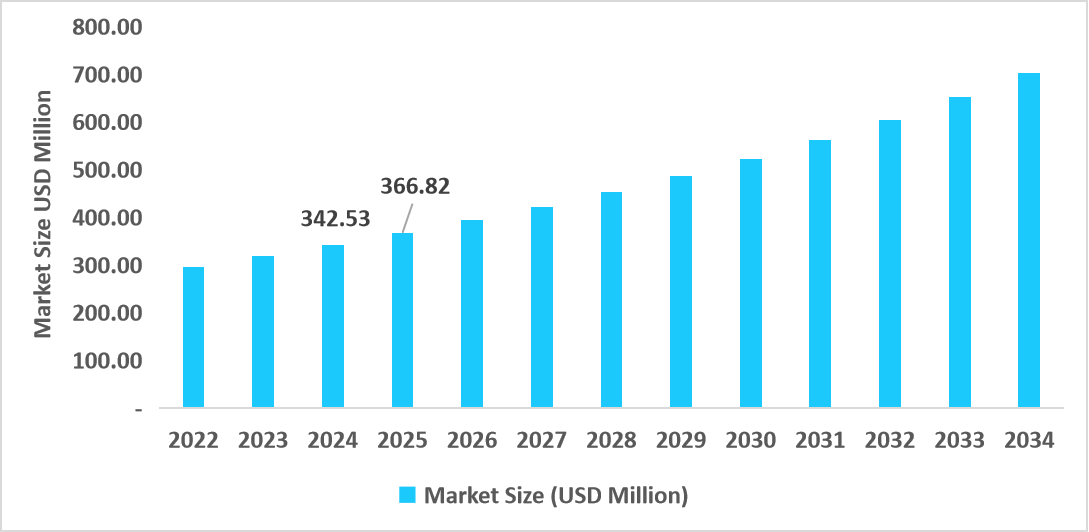
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.00 billion
- 2034 Projected Market Size: USD 1.92 billion
- CAGR (2026-2034): 7.52%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The antibody specificity testing market comprises a broad portfolio of products, technologies, and services designed to ensure accurate antibody performance across research and clinical settings. It includes antibodies, controls, kits, reagents, and gene validation tools, along with specialized testing services. Technologies span genetic validation, microarray platforms, and immunoassay-based methods such as Western blotting, immunochemistry, and flow cytometry. These solutions support applications in R&D and clinical diagnostics across diverse disease areas, serving pharmaceutical companies, academic institutes, and diagnostic laboratories.
Latest Market Trends
Growing adoption of genetic validation for high specificity antibody testing
A major trend in the antibody specificity testing market is the increasing shift from traditional assay-only validation methods toward advanced genetic validation technologies, such as CRISPR knockout and knockdown models. These tools enable researchers to confirm true target binding with higher accuracy and eliminate off-target interactions. As laboratories and biopharmaceutical companies prioritize reproducibility and precision, the demand for genetically validated antibody testing continues to rise, strengthening reliability across research and clinical applications.
Rising shift toward multiplex antibody validation platforms
A current trend in the antibody specificity testing market is the growing adoption of multiplex validation platforms that allow simultaneous assessment of multiple antibodies in a single workflow. Research organizations and biopharmaceutical companies are increasingly integrating high-throughput systems to evaluate binding specificity, cross-reactivity, and performance across several targets at once. This shift enhances efficiency, reduces reagent consumption, and accelerates development timelines, reflecting strong market demand for faster, scalable, and cost-effective antibody validation solutions.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.00 Billion |
| Estimated 2026 Value | USD 1.07 billion |
| Projected 2034 Value | USD 1.92 Billion |
| CAGR (2026-2034) | 7.52% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Abcam, Agilent Technologies, BD, Bio-Rad Laboratories, Bio-Techne Corporation |
to learn more about this report Download Free Sample Report
Antibody Specificity Testing Market Driver
Expanding use of Antibody-Based Therapeutics Drives Market Growth
The rapid global expansion of antibody-based therapeutics is emerging as a key growth driver for the market. As pharmaceutical and biotechnology companies increasingly develop monoclonal antibodies, bispecific antibodies, and targeted biologics, the necessity for highly accurate specificity testing has intensified. This rising therapeutic pipeline requires stringent validation to ensure safety and target accuracy, which boosts demand for advanced testing tools and services, thereby accelerating overall market growth.
Market Restraints
High cost of Advanced Antibody Validation Technologies
The high cost associated with advanced validation technologies and multi-platform testing protocols is restraining market growth. Comprehensive specificity assessment often requires genetic validation tools, high-resolution imaging systems, and multiple immunoassay formats, which increase operational expenses. For many small biotech firms and institutions in low and middle-income regions, these costs, combined with limited access to specialized equipment, restrict widespread adoption and slow market penetration globally.
Market Opportunities
Rising Demand for Antibody Validation in Emerging Biopharmaceutical Hubs
The rapid expansion of biopharmaceutical research in emerging markets is creating an opportunity for the antibody specificity testing market. Countries across Asia Pacific, including India, China, and South Korea, are investing heavily in biologics development, fueling demand for reliable antibody validation tools. For instance, several new biotech parks launched in 2025 incorporated dedicated antibody testing facilities to support therapeutic innovation. This growing research infrastructure is driving substantial uptake of advanced specificity testing solutions and services.
Regional Analysis
North America dominated the antibody specificity testing market in 2025, accounting for 39.83% market share. This dominance is attributed to the rapid expansion of antibody validation consortia within major U.S. biomedical clusters, where academic-industry collaborations employ advanced multiplex specificity platforms to standardize reagent performance.
In the U.S., the surge of antibody deconvolution programs funded by precision medicine initiatives, where national biobanks increasingly require orthogonal specificity verification. This demand for rigorously validated reagents supports rapid adoption of advanced specificity workflows, strengthening the domestic ecosystem for high-fidelity antibody characterization technologies across research and diagnostics, thereby supporting market growth.
Asia Pacific Market Insights
Asia Pacific is emerging as a fastest growing region with a CAGR of 9.18%. from 2026-2034. The market growth is propelled by the rapid establishment of antibody-repurposing hubs within national genomics missions, where large cohorts require culturally diverse biomarker panels. This pushes demand for localized specificity testing platforms capable of validating antibodies against region specific genetic variants, accelerating uptake of advanced characterization technologies across emerging biopharma and academic laboratories.
Japan antibody specificity testing market is experiencing notable growth, due to expanding monoclonal antibody validation programs, where long used research antibodies are re-benchmarked with next-generation specificity assays to support reproducibility mandates. This systematic revalidation effort increases demand for high precision orthogonal testing platforms, strengthening domestic adoption across pharmaceutical labs, national research institutes, and advanced translational medicine centers.
Regional Market share (%) in 2025
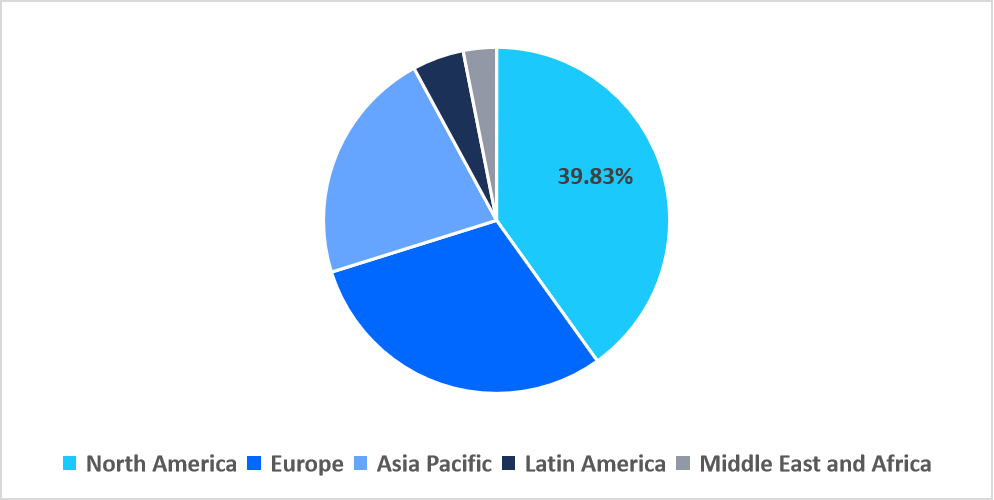
Source: Straits Research
Europe Market Insights
The Europe market is surging due to EU antibody harmonization frameworks embedded within biomedical infrastructures. These initiatives require reproducibility compliant specificity audits for antibodies used in multinational clinical cohorts, driving accelerated adoption of standardized validation platforms.
Growth of the antibody specificity testing market in Germany is supported by the rapid scaling of domestic antibody contract manufacturing (CMO) capacity, which fuels demand for in-house specificity validation. German CMOs expand commercial monoclonal antibody production for both local and export markets and increasingly invest in rigorous antibody specificity testing, which, in turn, supports market growth.
Latin America Market Insights
The Latin America market growth is shaped by Brazil’s expanding biosimilar and biologics manufacturing base, which has prompted stricter regulatory expectations for antibody characterization. This shift drives accelerated uptake of advanced, high-throughput specificity testing platforms to ensure quality, reproducibility, and compliance across emerging biopharmaceutical workflows.
The growth of the antibody specificity testing market in Argentina is stimulated by expanding biosimilar monoclonal antibody manufacturing capacity, supported by new GMP compliant production facilities, which drives the demand for stringent antibody quality verification. This development is accelerating demand for advanced specificity testing tools to ensure consistent performance, regulatory alignment, and reliability across the country’s growing biopharmaceutical pipeline.
Middle East and Africa Market Insights
The Middle East and Africa market is experiencing growth due to the launch of the Middle East’s first biologics manufacturing hub equipped with dedicated bioanalytical facilities for monoclonal antibody workflows, which intensified the demand for robust antibody specificity assessment.
Saudi Arabia’s development of a fully integrated GMP-compliant advanced therapy manufacturing campus spanning cell, gene, and biologics production intensified the requirement for stringent antibody specificity validation, accelerating the adoption of high-precision testing platforms across the nation.
Product & Services Insights
The product segment dominated the market in 2025. This growth is due to the rising demand for ultra-pure antibody reference standards used to calibrate advanced analytical instruments. As laboratories adopt high-resolution platforms, these precision-engineered standards have become essential for ensuring assay accuracy, driving strong and specialized product uptake.
The antibody validation & specificity testing services segment is projected to grow at the fastest CAGR of 8.49% during the forecast period, owing to the rising demand for rare epitope cross-verification, where antibodies targeting ultra-low-abundance or structurally concealed epitopes require specialized multi-step validation. Only advanced service providers offer this expertise, driving strong demand for outsourced precision testing.
Technology Insights
The immunoassay-based technologies segment accounted for the 51.63% market share in 2025, due to increased use of ultra sensitive chemiluminescent substrates designed specifically for detecting weak antibody antigen interactions. These next-generation substrates enhanced signal precision in low expression targets, making immunoassays the preferred platform for high-fidelity specificity testing across research settings.
The genetic validation-based technologies segment is expected to register the fastest CAGR of 8.72% during the forecast period. This growth is propelled by the rising adoption of CRISPR-dual knockout models specifically designed to verify antibody selectivity between closely related isoforms. This advanced approach eliminates ambiguity in target confirmation, making genetic validation indispensable for high-precision antibody testing.
By Technology Market Share (%), 2025
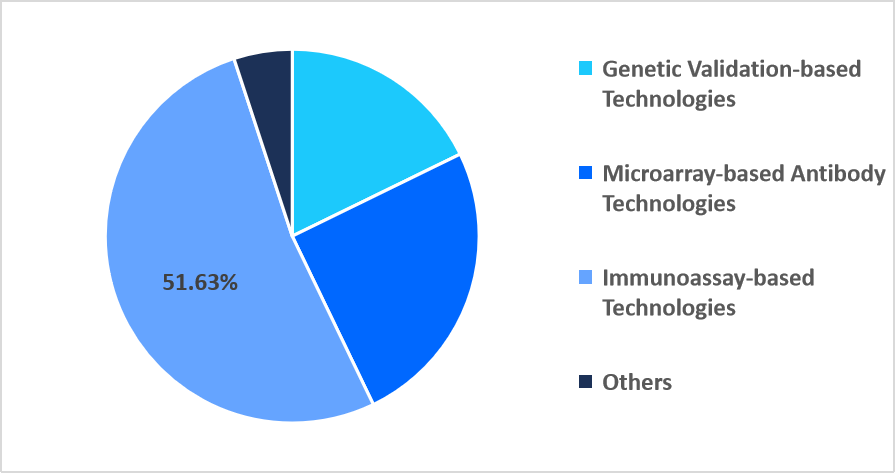
Source: Straits Research
Application Insights
The research & development segment dominated the market in 2025, due to growing reliance on pre-clinical antibody de-risking programs, where labs increasingly validate specificity early to avoid downstream clinical failures. This shift toward upfront molecular verification notably increased demand for sophisticated R&D-focused antibody testing workflows.
The clinical diagnostics segment is expected to grow at a CAGR of 8.65% during the forecast period, due to the adoption of microfluidic enabled antibody cross reactivity screens, which allow clinicians to assess specificity using minimal sample volumes. This advancement supports faster diagnostic turnaround and enhances accuracy, prompting broader clinical integration of antibody specificity testing.
End Use Insights
The pharmaceutical & biotechnology companies segment dominated the market with a revenue share of 53.06% in 2025. This growth is driven by the integration of digital twin antibody interaction modeling platforms within pharma R&D workflows. These systems simulate real-time antibody target behavior, enabling companies to pre-validate specificity before laboratory testing. This reduces development risks and accelerates biologics pipelines, boosting segment dominance.
Competitive Landscape
The global antibody specificity testing market presents a moderately competitive landscape, characterized by a blend of established biotechnology corporations and specialized validation service providers. Leading players leverage advanced analytical platforms, proprietary assay technologies, and large-scale R&D capabilities to maintain their market position. Companies such as Thermo Fisher Scientific, Abcam, Bio-Rad Laboratories, Cell Signaling Technology, and Rockland Immunochemicals focus on product innovation, strategic partnerships, and expanded validation services to meet rising demand for high-accuracy antibody performance across research and clinical applications.
BioVeritas Labs: An emerging market player
BioVeritas Labs is an emerging biotechnology company gaining traction in the global market through its next-generation microfluidic validation platforms. The company focuses on ultra-high-throughput antibody screening, enabling rapid detection of cross-reactivity issues at early research stages. Its automated cartridge-based testing system, launched in 2025, offers improved accuracy and reduced assay time. By integrating advanced analytics and compact instrumentation, BioVeritas is quickly establishing itself as a competitive new entrant in the global market.
List of Key and Emerging Players in Antibody Specificity Testing Market
- Abcam
- Agilent Technologies
- BD
- Bio-Rad Laboratories
- Bio-Techne Corporation
- Cell Signaling Technology
- Creative Biolabs
- Creative Diagnostics
- Danaher Corporation
- Hoffmann-La Roche Ltd.
- GenScript Biotech Corporation
- Luminex Corporation
- Merck KGaA
- Novus Biologicals
- OriGene Technologies
- PerkinElmer
- Rockland Immunochemicals
- Sino Biological
- Thermo Fisher Scientific
- Others
Strategic Initiatives
- June 2025: Bio-Rad Laboratories, Inc., expanded its range of recombinant monoclonal anti-idiotypic antibodies with the introduction of antibodies specific to pertuzumab, guselkumab, canakinumab, belimumab, and the bispecific drug emicizumab.
- March 2024: Bio-Rad Laboratories, Inc. launched the validated antibodies for rare cell and circulating tumor cell (CTC) enumeration.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.00 Billion |
| Market Size in 2026 | USD 1.07 billion |
| Market Size in 2034 | USD 1.92 Billion |
| CAGR | 7.52% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product & Services, By Technology, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Antibody Specificity Testing Market Segments
By Product & Services
-
Products
- Antibodies
- Control & Standards
- Kits & Reagents
- Gene Validation Tools
- Antibody Validation & Specificity Testing Services
By Technology
- Genetic Validation-based Technologies
- Microarray-based Antibody Technologies
- Immunoassay-based Technologies
- Western Blotting
- Immunochemistry
- Flow Cytometry
- Others
By Application
- Research & Development
-
Clinical Diagnostics
- Infectious Diseases
- Oncology
- Immunology & Autoimmune Disorders
- Neurodegenerative Disorders
- Metabolic Disorders
- Others
By End Use
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Diagnostic Laboratories
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































