Antimicrobial Resistance Diagnostics Market Size, Share & Trends Analysis Report By Technology (Microbial Culture, Immunoassay, Polymerase Chain Reaction, Next-generation Sequencing, Mass Spectrometry, Others), By Pathogen (Methicillin-Resistant Staphylococcus Aureus, Drug-Resistant Streptococcus Pneumoniae, Drug-Resistant Campylobacter, Drug-Resistant Neisseria Gonorrhea, Drug-Resistant Salmonella, Others), By End Use (Hospitals, Diagnostic Laboratories, Pharmaceutical & Biotechnology Companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Antimicrobial Resistance Diagnostics Market Overview
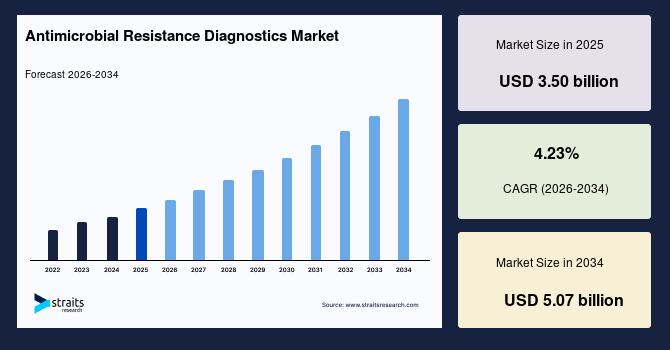
The global antimicrobial resistance diagnostics market size is valued at USD 3.50 billion in 2025 and is estimated to reach USD 5.07 billion by 2034, growing at a CAGR of 4.23% during the forecast period. The market growth is accelerated by the integration of hospital antibiograms with AI-supported prescribing systems, forcing routine resistance testing to guide real-time antibiotic selection decisions.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 37.84% share in 2025.
- The Asia Pacific region is forecasted to grow at the fastest pace, with a CAGR of 5.83%during the forecast timeframe.
- Based on technology, the microbial culture segment dominated the market in 2025, accounting for 51.54% revenue share.
- Based on pathogen, the drug-resistant Neisseria gonorrhoeae segment is expected to register the fastest CAGR of 5.17% during 2026-2034.
- By end use, the hospitals segment dominated the market, accounting for 59.04% revenue share in 2025.
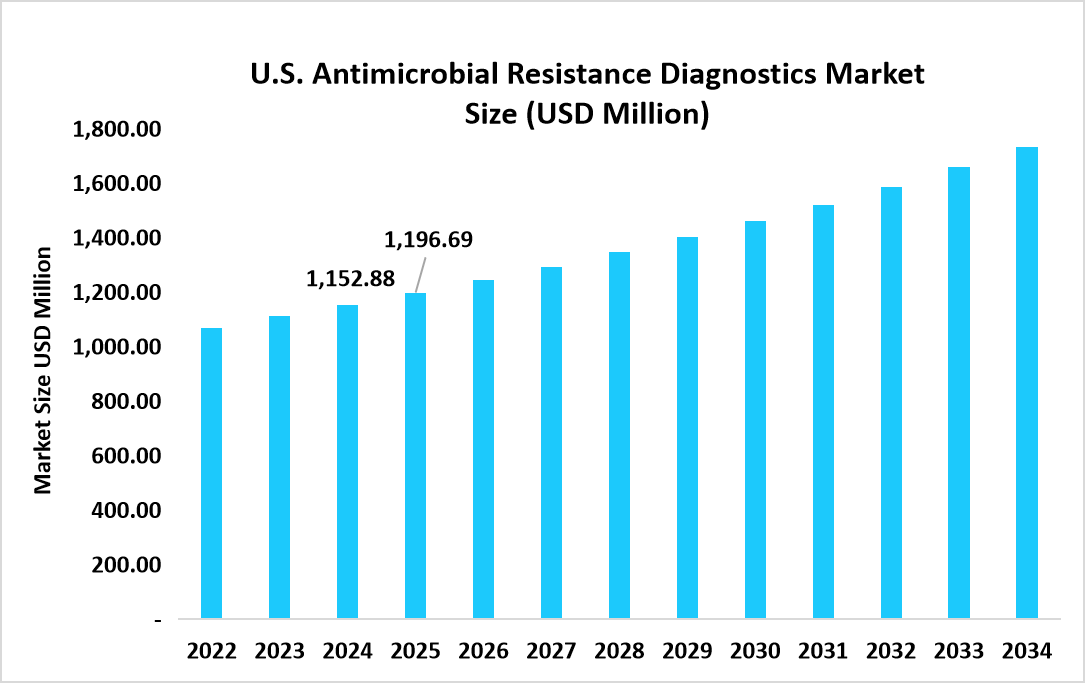
- The U.S. dominates the market, valued at USD 1.15 billion in 2024 and reaching USD 1.19 billion in 2025.
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 3.50 billion
- 2034 Projected Market Size: USD 5.07 billion
- CAGR (2026-2034): 4.23%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The antimicrobial resistance diagnostics market comprises diagnostic technologies, such as microbial culture, immunoassays, PCR, next-generation sequencing, mass spectrometry, and other advanced tools to detect resistant organisms such as MRSA, drug-resistant Streptococcus pneumoniae, Salmonella, Neisseria gonorrhoeae, and others. These diagnostics are widely utilized across hospitals, diagnostic laboratories, pharmaceutical and biotechnology companies, and other healthcare settings.
Market Trends
Emergence of Rapid & Portable Resistance Testing Platforms
The widespread adoption of rapid, point-of-care molecular and resistance marker tests is a key trend for market growth. Manufacturers such as Thermo Fisher Scientific, Roche, Abbott, and Cepheid have launched PCR based and cartridge-based assays that substantially reduce turnaround times and support immediate clinical decision making, expanding decentralized AMR testing beyond traditional laboratories.
This shift toward portable diagnostics enhances antibiotic stewardship, improves patient outcomes, and drives broader market uptake globally.
Expansion of Multiplex PCR & Integrated Resistance Panels
A major trend in the antimicrobial resistance diagnostics market is the rapid development and adoption of multiplex PCR assays that detect multiple pathogens and resistance genes simultaneously, improving diagnostic speed and accuracy. For example, Vela Diagnostics’ PathoKey MP UTI ID & AMR PCR Test identifies 14 urinary pathogens and 14 resistance markers in about four hours, allowing faster clinical decisions. This multi-target technology is driving broader laboratory adoption and enhancing antimicrobial stewardship worldwide.
Such PCR platforms are becoming central to AMR diagnostics, boosting clinical efficiency and market growth.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 3.50 billion |
| Estimated 2026 Value | USD 3.64 billion |
| Projected 2034 Value | USD 5.07 billion |
| CAGR (2026-2034) | 4.23% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | bioMérieux SA, Hoffmann-La Roche AG, Thermo Fisher Scientific Inc., Abbott, Danaher |
to learn more about this report Download Free Sample Report
Market Driver
Increasing Prevalence of Drug-Resistant Infections
A major driver in the antimicrobial resistance diagnostics market is the global rise in drug-resistant infections, compelling healthcare systems to adopt advanced diagnostic solutions for early, precise detection and targeted treatment. For instance, the CDC reports AMR causes over 2.8 million infections annually in the U.S., accelerating demand for rapid PCR, NGS, and point-of-care resistance assays.
The urgent requirements to relieve morbidity, improve outcomes, and guide effective antimicrobial therapy are boosting market growth for AMR diagnostic technologies.
Graph: Antibiotic Resistance Cases Growth Between 2018-2024
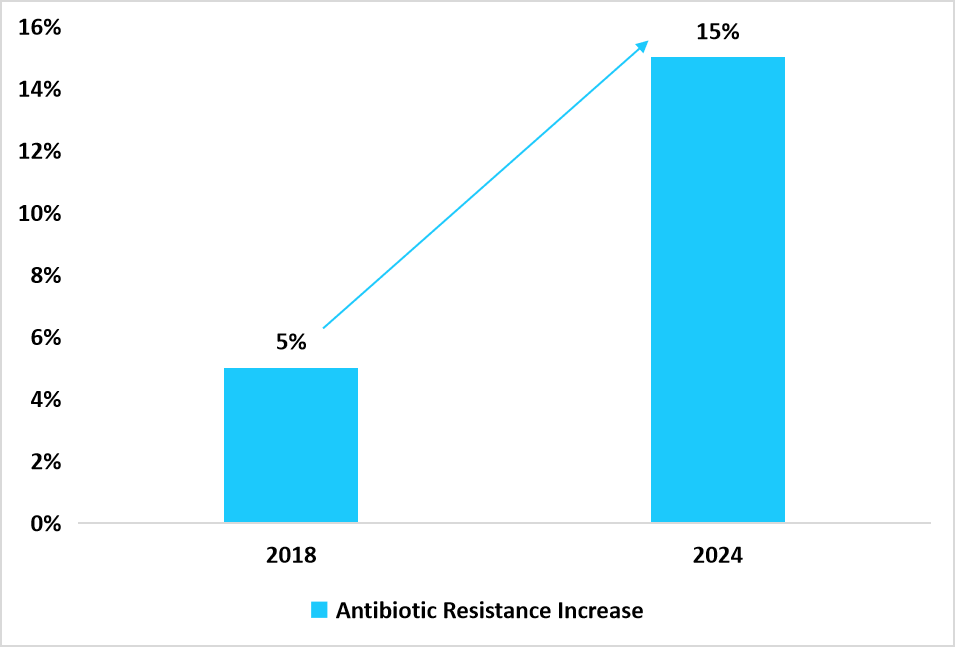
Source: UNICEF
Market Restraint
High Cost And Limited Accessibility of Advanced AMR Diagnostics
The high cost and limited accessibility of advanced diagnostic technologies, which restrict widespread clinical adoption, are restraining the market growth. Molecular and next-generation platforms require high capital investment, ongoing reagent expenses, and skilled personnel in low and middle-income regions where budgets are constrained, and reimbursement frameworks are weak. These economic and infrastructure hurdles reduce uptake despite critical clinical necessities, slowing overall market growth and implementation.
Market Opportunity
Expansion of AI-enabled and Genomic AMR Surveillance Platforms
The expansion of AI-driven analytics, combined with next-generation sequencing for AMR surveillance. Leading manufacturers such as bioMérieux and Thermo Fisher Scientific have highlighted, in investor presentations, growing demand for genomic surveillance tools that integrate laboratory data with hospital and public health networks. These solutions enable early outbreak detection, guide stewardship programs, and support population-level resistance monitoring.
Regional Analysis
North America dominated the market in 2025, accounting for 37.84% market share. This growth is supported by the CDC’s extensive Antimicrobial Resistance Laboratory Network, which supports advanced resistance detection and whole genome sequencing across all 50 states, enabling rapid outbreak identification and high‑resolution resistance tracking that fuels demand for sophisticated diagnostics.
In U.S., the market growth is supported by non‑dilutive funding provided through the CARB‑X accelerator, supported by BARDA and NIH, which has awarded millions to U.S. diagnostics developers such as Pattern Bioscience and Day Zero Diagnostics to advance rapid AMR tests, combining genomics and machine learning for faster pathogen and resistance profiling. This strategic investment accelerates commercialization and strengthens U.S. diagnostic innovation pipelines.
Asia Pacific Antimicrobial Resistance Diagnostics Market Insights
Asia Pacific is emerging as a fastest growing region with a CAGR of 5.83% from 2026-2034, owing to India’s development of localized AMR genomic benchmarks, where institutions like AIIMS Mangalagiri are establishing indigenous epidemiological cut‑off values (ECOFFs) and building state‑of‑the‑art genomic AMR databases tailored to local bacterial resistance profiles. This initiative enhances diagnostic accuracy and accelerates meaningful AMR data integration into clinical practice across Asia.
Australia's antimicrobial resistance diagnostics industry is gaining momentum with the HOTspots pilot program within the national AURA surveillance system, which delivers regional antimicrobial resistance data to clinicians in remote and rural northern Australia. By providing local AMR trend insights from distributed pathology providers, HOTspots allow diagnostic decision-making and strengthen demand for advanced resistance assays in underserved regions.

Source: Straits Research
Europe Market Insights
Europe antimicrobial resistance diagnostics market growth is supported by the European Partnership on One Health AMR initiative, which unites 53 organizations from 30 countries with more than USD 250 million in pooled research and innovation funding to accelerate One Health AMR diagnostic solutions and surveillance tools. This structured pan‑EU collaboration enhances development, harmonizes standards, and drives uptake of advanced diagnostics across Europe.
Germany's market growth is augmented by the development and rollout of the ARVIA platform by the Robert Koch Institute, which integrates antibiotic resistance and consumption data electronically from hospitals and outpatient labs for benchmarking, feedback reports, and tailored diagnostic decision support that enhances resistance testing across German healthcare facilities.
Latin America Market Insights
The growth of antimicrobial resistance diagnostics market in Latin America is supported by the development of regionally tailored digital tools, such as Argentina’s AntibioticApp, which provides healthcare professionals with updated, locally relevant antimicrobial resistance and prescribing guidance.
Brazil’s market growth is stimulated by local research into rapid lateral flow assays for detecting multidrug-resistant bacteria, such as projects funded by FAPEMAT that implement low-cost, point‑of‑care lateral flow tests, reducing turnaround time and enabling broader resistance testing in both clinical and veterinary settings.
Middle East and Africa Market Insights
The Middle East and Africa region is experiencing an Africa CDC- supported AMR & AMU Partnership consortium, which, for the first time, aggregated over 819,500 antimicrobial resistance data records from 205 laboratories across 14 African countries, allowing region-specific resistance profiling and strengthening diagnostic demand through policy and lab strategy alignment.
Egypt’s market growth is driven by the government’s rollout of an electronic antimicrobial resistance surveillance system in partnership with Axis Pharma, designed to digitize and integrate national AMR data across hospitals and laboratories, improving visibility of resistance patterns and supporting diagnostic and antibiotic policy decisions.
Technology Insights
The microbial culture segment dominated the market, accounting for a revenue share of 51.54% in 2025. This growth is driven by the development of advanced chromogenic and selective culture media, such as Thermo Fisher Scientific’s Oxoid Brilliance ESBL Agar, designed to improve detection and differentiation of extended-spectrum β-lactamase-producing Enterobacteriaceae and other resistant bacteria, increasing laboratory confidence in phenotypic resistance identification.
The next-generation sequencing segment is projected to grow at the fastest CAGR of 5.07% during the forecast period, due to the use of hybrid capture-based targeted NGS assays that enrich antimicrobial resistance genes even when present at very low abundance in clinical samples, improving sensitivity over untargeted methods.
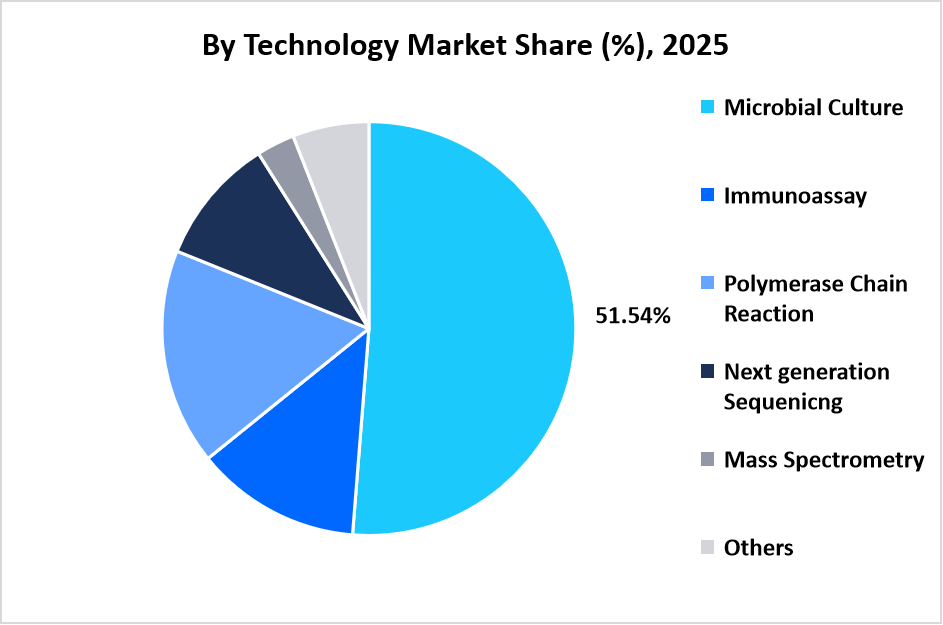
Source: Straits Research
Pathogen Insights
The Methicillin resistant staphylococcus aureus segment dominated the market, accounting for 21.15% in 2025. This dominance is augmented by the widespread clinical adoption of rapid, PCR-based blood culture assays like Cepheid’s Xpert MRSA/SA, which deliver detection of MRSA directly from positive blood cultures, substantially shortening time to targeted therapy compared with conventional methods.
The drug-resistant Neisseria gonorrhoeae segment is estimated to register the fastest CAGR of 5.17% during the forecast period, owing to the commercialization of multiplex PCR assays like Roche’s ResistancePlus GC test, which detects N. gonorrhoeae and specific gyrA mutations linked to ciprofloxacin resistance in a single assay for precise resistance profiling directly from clinical specimens and improving targeted treatment decisions.
End Use Insights
The hospitals segment dominated the market, accounting for 59.04% market share in 2025, due to the integration of advanced automated microbiology systems like BD’s Phoenix M50 with hospital informatics, enabling rapid antimicrobial resistance detection and seamless delivery of actionable results to clinicians.
The diagnostic laboratories segment is projected to grow at the fastest CAGR during 2026-2034, owing to increasing requirements for specialized proficiency testing and ISO/CLIA accreditation for AMR diagnostics, prompting labs to adopt advanced resistance assays and quality‑assured platforms to meet stringent regulatory and referral standards.
Competitive Landscape
The global antimicrobial resistance diagnostics market is moderately consolidated, with key players including bioMérieux, Thermo Fisher Scientific, Roche, Abbott, Danaher, Becton Dickinson, and others dominating the landscape. These companies focus on developing rapid molecular assays, multiplex PCR panels, and advanced phenotypic AST systems, while actively pursuing strategic collaborations, acquisitions, and regional expansions. Continuous R&D investments aim to enhance diagnostic accuracy, reduce turnaround times, and broaden geographic presence, ensuring a competitive advantage in the growing AMR diagnostics sector.
OpGen Inc.: An emerging market player
OpGen Inc. is an emerging company in the market, focusing on advanced genomic and informatics-based solutions for rapid detection of resistant pathogens. Recently, OpGen has expanded its Acuitas AMR Gene Panel and surveillance software for hospitals and laboratories to identify resistance genes and track outbreaks. Such strategic collaborations and technology integrations position OpGen as a growing player addressing the increasing demand for AMR diagnostics globally.
List of Key and Emerging Players in Antimicrobial Resistance Diagnostics Market
- bioMérieux SA
- Hoffmann-La Roche AG
- Thermo Fisher Scientific Inc.
- Abbott
- Danaher
- BD
- QIAGEN N.V.
- Sysmex Corporation
- Bio-Rad Laboratories Inc.
- Accelerate Diagnostics Inc.
- Alifax S.r.l.
- Lumos Diagnostics Holdings Pty Ltd
- Molsid SAS
- Genetic Signatures Limited
- iFAST Diagnostics Ltd
- Astek Diagnostics Inc.
- OpGen Inc.
- T2 Biosystems, Inc.
- Others
Strategic Initiatives:
- March 2025: bioMérieux, a leading developer of IVD, received FDA approval for its VITEK COMPACT PRO, a system for microorganism identification and antibiotic susceptibility testing.
- July 2025:The Centre for Cellular and Molecular Platforms, with the support of the International Centre for Antimicrobial Resistance Solutions (ICARS), launched the One Health AMR Challenge 2025 to advance technologies tackling antimicrobial resistance.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 3.50 billion |
| Market Size in 2026 | USD 3.64 billion |
| Market Size in 2034 | USD 5.07 billion |
| CAGR | 4.23% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Technology, By Pathogen, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Antimicrobial Resistance Diagnostics Market Segments
By Technology
- Microbial Culture
- Immunoassay
- Polymerase Chain Reaction
- Next-generation Sequencing
- Mass Spectrometry
- Others
By Pathogen
- Methicillin-Resistant Staphylococcus Aureus
- Drug-Resistant Streptococcus Pneumoniae
- Drug-Resistant Campylobacter
- Drug-Resistant Neisseria Gonorrhea
- Drug-Resistant Salmonella
- Others
By End Use
- Hospitals
- Diagnostic Laboratories
- Pharmaceutical & Biotechnology Companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































