Antimicrobial Susceptibility Testing Market Size, Share & Trends Analysis Report By Product Type (Manual AST Products, Automated AST Instruments, Consumables, Software & Services), By Techniques (Automated AST, Etest Method, Dilution, Disk Diffusion, Others), By Application (Drug Development, Susceptibility Testing, Others), By End-user (Hospitals, Diagnostic Laboratories, Biotechnology & Pharmaceutical Companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Antimicrobial Susceptibility Testing Market Size
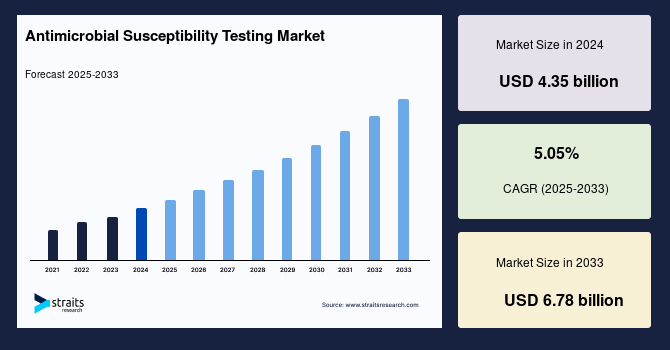
The global antimicrobial susceptibility testing market size was valued at USD 4.35 billion in 2024 and is projected to grow from USD 4.57 billion in 2025 to USD 6.78 billion by 2033, growing at a CAGR of 5.05% during the forecast period (2025–2033).
The global antimicrobial susceptibility testing (AST) market is primarily driven by the rising prevalence of antimicrobial resistance (AMR), which has become a critical global health threat. As traditional antibiotics lose effectiveness, the need for accurate and timely AST solutions has intensified to guide appropriate therapeutic decisions. Government initiatives and global health organizations, such as the WHO and CDC, are increasingly investing in surveillance programs and promoting the use of AST to curb the spread of resistant infections.
In addition, the growing emphasis on antimicrobial stewardship programs across healthcare systems is further fueling market demand. These programs aim to optimize the use of antimicrobials through evidence-based practices in which susceptibility testing plays a vital role. Moreover, the pharmaceutical industry's ongoing research and development efforts for novel antibiotics also contribute to market growth, as new drug candidates require extensive susceptibility profiling. Together, these factors are driving the increased adoption and development of AST methods across the globe.
Current Market Trends
Integration of Ai and Machine Learning
A key trend shaping the global Antimicrobial Susceptibility Testing (AST) market is the integration of artificial intelligence (AI) and machine learning (ML) to enhance diagnostic precision and reduce turnaround times. These technologies analyze large-scale clinical and genomic datasets to detect resistance patterns and recommend effective treatment options.
- For instance, in April 2024, the ASTar platform by Q-linea, a Swedish company, secured U.S. FDA 510(k) clearance, offering <7-hour antimicrobial susceptibility testing (AST) using high-resolution time-lapse microscopy powered by machine-learning analysis. It demonstrated 95–98% categorical agreement in clinical evaluations.
This innovation signifies a shift from conventional, time-consuming AST methods to AI-driven systems that accelerate decision-making in clinical microbiology. As AMR continues to rise, the adoption of AI/ML-enabled AST platforms is expected to become increasingly vital in guiding targeted antibiotic therapy and strengthening antimicrobial stewardship programs worldwide.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 4.35 Billion |
| Estimated 2025 Value | USD 4.57 Billion |
| Projected 2033 Value | USD 6.78 Billion |
| CAGR (2025-2033) | 5.05% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | bioMérieux SA, Becton, Dickinson and Company, Thermo Fisher Scientific Inc., Danaher Corporation, Bio-Rad Laboratories Inc. |
to learn more about this report Download Free Sample Report
Antimicrobial Susceptibility Testing Market Drivers
Increase in Infectious Disease Outbreaks, Including Hospital-Acquired Infections (hais)
A key driver of the global antimicrobial susceptibility testing (AST) market is the growing prevalence of infectious disease outbreaks, including hospital-acquired infections (HAIs). The emergence and robust spread of resistant pathogens in clinical settings have increased the demand for accurate diagnostic tools to guide effective treatment.
- According to the World Health Organization, approximately 136 million HAIs occur worldwide each year, with around 119 million reported in low- and middle-income countries. In the United States alone, the Centers for Disease Control and Prevention (CDC) estimates that about 1 in 31 hospital patients has at least one HAI on any given day.
These alarming figures underscore the need for timely AST to control infection spread and improve patient outcomes. Thus, the increased focus on infection prevention and antimicrobial stewardship further supports the expansion of the AST market globally.
Market Restraining Factors
High Cost of Automated Ast Systems
One of the major restraints hindering the growth of the global antimicrobial susceptibility testing (AST) market is the high cost associated with automated AST systems. These advanced systems, while offering improved accuracy and faster turnaround times, require substantial capital investment and ongoing maintenance.
This poses a prominent challenge for healthcare facilities in low- and middle-income countries, where budget constraints often limit the adoption of high-end diagnostic technologies. Additionally, the cost of reagents, consumables, and training further adds to the financial burden. As a result, many small-scale laboratories and clinics continue to rely on conventional, manual methods, thereby limiting the widespread implementation of automated AST solutions.
Market Opportunities
Development of Rapid, Point-of-Care Ast Solutions
The development of rapid, point-of-care (POC) antimicrobial susceptibility testing (AST) solutions offers a transformative opportunity in the fight against antimicrobial resistance. Traditional AST methods often require 24–72 hours, delaying targeted treatment and increasing reliance on broad-spectrum antibiotics.
- In June 2023, Sysmex Europe introduced the world’s first POC AST system capable of delivering susceptibility results from urine samples in as little as 30 minutes a dramatic reduction from the conventional 48–72 hours. It utilizes proprietary microfluidic technology, enabling automatic bacterial detection (within ~15 minutes) and antibiotic effectiveness profiling all within a compact desktop device for near-patient use.
Such innovations improve clinical outcomes by enabling timely, evidence-based prescriptions while reducing hospital stays. As healthcare systems increasingly prioritize rapid diagnostics, the demand for decentralized, efficient AST tools is expected to grow significantly across global markets.
Regional Insights
The antimicrobial susceptibility testing market in North America is witnessing robust growth due to the strong presence of advanced healthcare infrastructure and the extensive implementation of antimicrobial stewardship programs. High awareness regarding antimicrobial resistance and increased investment in diagnostic innovation are fueling demand for rapid and automated AST solutions. The region also benefits from a well-established regulatory framework that supports the commercialization of novel testing technologies. Ongoing collaborations between research institutions and diagnostic firms further enhance the development and deployment of next-generation susceptibility testing tools across clinical and research settings.
U.s. Antimicrobial Susceptibility Testing Market Trends
- The U.S. market is driven by CDC-led initiatives like the Antibiotic Resistance (AR) Solutions Initiative. Leading hospitals and labs deploy automated systems such as Thermo Fisher’s Sensititre and bioMérieux’s VITEK 2. The FDA’s fast-tracking of AST device approvals and rising incidences of multidrug-resistant organisms, like CRE (carbapenem-resistant Enterobacteriaceae), boost the adoption of advanced AST technologies across healthcare settings nationwide.
- Canada’s antimicrobial susceptibility testing market is growing due to national efforts like the Canadian Antimicrobial Resistance Surveillance System (CARSS). Hospitals increasingly adopt rapid AST tools, such as VITEK 2 and BD Phoenix systems, to manage rising drug-resistant infections. Government funding and public health campaigns promoting antimicrobial stewardship further support the demand for AST solutions in clinical microbiology labs and reference centers across provinces like Ontario and British Columbia.
Asia-Pacific Antimicrobial Susceptibility Testing Market Trends
Asia Pacific is experiencing accelerated growth in the market for antimicrobial susceptibility testing, propelled by rising healthcare investments and increasing awareness about antimicrobial resistance. The region is seeing a surge in demand for affordable and rapid diagnostic solutions in both urban and rural healthcare settings. Government initiatives aimed at improving infection control and diagnostic capacity are fostering market expansion. Additionally, the growing burden of infectious diseases, coupled with the gradual adoption of modern laboratory practices and technologies, is driving the uptake of AST tools across hospitals, reference labs, and academic research institutes.
- China’s antimicrobial susceptibility testing market is expanding due to rising AMR cases and strong government-led healthcare reforms. The “Healthy China 2030” initiative emphasizes improved diagnostic capabilities. For example, hospitals in Beijing and Shanghai have adopted automated AST systems to reduce HAIs. Additionally, collaborations with international players like bioMérieux are enhancing local testing capabilities, supporting broader adoption across public and private healthcare institutions.
- India’s antimicrobial susceptibility testing industry is growing rapidly, driven by high infectious disease burdens and national programs like the AMR Surveillance Network led by ICMR. For instance, AIIMS and other major hospitals are implementing automated AST systems to manage multidrug-resistant infections. The Indian government's “National Action Plan on AMR” supports infrastructure upgrades and training, creating demand for reliable susceptibility testing solutions, especially in urban tertiary care centers.
Europe Antimicrobial Susceptibility Testing Market Trends
Europe's market for antimicrobial susceptibility testing is driven by stringent healthcare regulations promoting antibiotic surveillance and stewardship. The region emphasizes responsible antibiotic use through national and transnational health initiatives, boosting demand for AST in clinical diagnostics. Integration of AST systems into public healthcare facilities and laboratories is accelerating, supported by consistent funding for infectious disease management. Additionally, the growing focus on precision medicine and increased adoption of laboratory automation technologies are enhancing the efficiency and scalability of susceptibility testing processes across hospitals, diagnostic labs, and pharmaceutical research centers in the region.
- Germany's antimicrobial susceptibility testing market is driven by strong healthcare infrastructure and strict AMR control policies. The Robert Koch Institute promotes advanced diagnostic adoption, such as VITEK 2 systems in clinical labs. Germany's National Antibiotic Resistance Strategy (DART) encourages AST integration in public hospitals. Moreover, the presence of major players like Merck KGaA and Siemens Healthineers supports the innovation and deployment of automated AST platforms nationwide.
- The UK antimicrobial susceptibility testing market benefits from NHS-backed AMR surveillance programs and public health initiatives. The UK Health Security Agency (UKHSA) supports routine AST implementation, using systems like BD Phoenix in NHS laboratories. The UK’s "Tackling AMR" national action plan emphasizes rapid diagnostics and stewardship. Additionally, collaborations with research hubs like the University of Oxford foster the development of novel AST tools and genetic resistance profiling.
Product Type Insights
Manual AST products hold a dominant share in the market due to their cost-effectiveness, ease of use, and widespread adoption in low- and middle-income regions. Products such as MIC strips, susceptibility testing discs, and culture media are essential in routine microbiology laboratories, especially where access to automated systems is limited. These tools offer reliable results for identifying effective antimicrobial agents against pathogens. The versatility and affordability of manual methods make them indispensable in both clinical and research settings, contributing to their sustained demand and dominance in the overall AST market.
Techniques Insights
The disk diffusion method remains the dominant technique in the market owing to its simplicity, low cost, and proven effectiveness. It is widely used in clinical laboratories for its ability to provide clear, interpretable results without the need for advanced instrumentation. This method is especially valuable in resource-constrained environments where automated systems are not feasible. Additionally, disk diffusion is recommended by various clinical guidelines, enhancing its credibility and global acceptance. Its consistent performance in detecting resistance patterns across a broad range of pathogens solidifies its leading position in AST techniques.
Application Insights
Susceptibility testing leads the application segment due to its central role in guiding effective antimicrobial therapy. As antimicrobial resistance continues to rise, healthcare providers rely on susceptibility testing to identify the most appropriate antibiotics for individual patients. This application is critical in managing hospital-acquired infections, optimizing treatment regimens, and reducing the spread of resistant strains. The increasing emphasis on evidence-based treatment and antimicrobial stewardship programs further drives the demand for susceptibility testing. Its essential function in both clinical diagnostics and public health surveillance positions it as the most significant application in the AST market.
End-User Insights
Hospitals represent the dominant end-user segment in the market due to the high volume of patient admissions and associated infectious disease cases. These healthcare settings require reliable AST tools to promptly identify resistance profiles and ensure accurate, timely treatment. The growing prevalence of multidrug-resistant infections in hospital environments, such as intensive care units (ICUs), necessitates routine AST to guide therapeutic decisions. Additionally, hospitals often have the infrastructure and trained personnel to implement both manual and automated AST methods, making them primary adopters of advanced diagnostic solutions and sustaining their lead in this market.
Company Market Share
Companies in the antimicrobial susceptibility testing market are focusing on enhancing their product portfolios through the development of rapid, automated, and cost-effective testing solutions. They are investing in research and development to introduce innovative technologies, such as molecular diagnostics and AI-based platforms. Additionally, strategic collaborations, regulatory approvals, and global expansion efforts particularly in emerging markets are helping them strengthen their market position and cater to the surging demand for advanced diagnostic tools.
Becton, Dickinson and Company
Becton, Dickinson and Company (BD) is a leading global medical technology firm headquartered in the U.S., known for its innovations in diagnostics, medical devices, and biosciences. In the antimicrobial susceptibility testing (AST) market, BD plays a pivotal role through its BD Phoenix™ and BD BACTEC™ systems, which offer automated and rapid testing solutions. The company focuses on combating antimicrobial resistance by enhancing laboratory efficiency, accuracy, and speed in detecting resistant pathogens, supporting global healthcare efforts against infectious diseases.
- In May 2025, Becton, Dickinson and Company (BD) announced a significant investment of US$2.5 billion aimed at expanding its U.S. manufacturing capacity over the next five years. This move is part of BD's broader strategic initiative to strengthen its supply chain resilience, enhance production efficiency, and meet the surging global demand for advanced diagnostic and antimicrobial susceptibility testing (AST) solutions.
List of Key and Emerging Players in Antimicrobial Susceptibility Testing Market
- bioMérieux SA
- Becton, Dickinson and Company
- Thermo Fisher Scientific Inc.
- Danaher Corporation
- Bio-Rad Laboratories Inc.
- HiMedia Laboratories
- Liofilchem S.r.l.
- Creative Diagnostics
- Merck KGaA
- Accelerate Diagnostics Inc.

to learn more about this report Download Market Share
Recent Developments
- April 2025- The FDA granted 510(k) clearance for Becton Dickinson’s BD Phoenix M50 combined with the BDXpert System within the BD Synapsys Informatics suite. It incorporates advanced algorithms to interpret microbial identification (ID) and AST data, enhancing lab efficiency and speeding the delivery of accurate, clinically actionable results.
- March 2025- bioMérieux's VITEK® COMPACT PRO system received 510(k) clearance from the U.S. FDA. This integrated system for microorganism identification and antibiotic susceptibility testing (AST) is intended for use in both clinical and industrial laboratories. It supports infection diagnosis and efforts to address antimicrobial resistance by delivering faster, more precise, and consistent results than earlier VITEK® models.
- January 2025- FASTinov introduced a rapid Antimicrobial Susceptibility Testing (AST) solution capable of delivering results within just two hours. Utilizing a phenotypic approach based on flow cytometry, this innovative method quickly determines whether bacterial strains are susceptible, intermediate, or resistant to antibiotics.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 4.35 Billion |
| Market Size in 2025 | USD 4.57 Billion |
| Market Size in 2033 | USD 6.78 Billion |
| CAGR | 5.05% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Techniques, By Application, By End-user |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Antimicrobial Susceptibility Testing Market Segments
By Product Type
-
Manual AST Products
- MIC Strips
- Susceptibility Testing Discs
- Culture Media
- Automated AST Instruments
-
Consumables
- Reagents
- Panels & Plates
- Software & Services
By Techniques
- Automated AST
- Etest Method
- Dilution
- Disk Diffusion
- Others
By Application
- Drug Development
- Susceptibility Testing
- Others
By End-user
- Hospitals
- Diagnostic Laboratories
- Biotechnology & Pharmaceutical Companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































