Antinuclear Antibody Test Market Size, Share & Trends Analysis Report By Product (Reagents & Assay Kits, Systems, Software & Services), By Technique (ELISA, Immunofluorescence Assay, Multiplex Assay), By Application (Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjogren’s Syndrome, Scleroderma, Other Diseases), By End Use (Hospitals, Clinical Laboratories, Physician Office Laboratories, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Antinuclear Antibody Test Market Overview
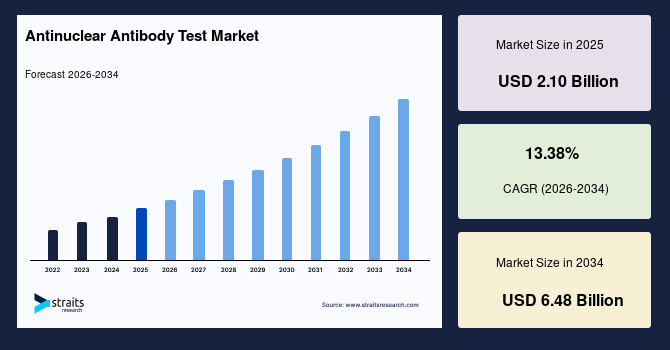
The global antinuclear antibody test market size is estimated at USD 2.10 billion in 2025 and is projected to reach USD 6.48 billion by 2034, growing at a CAGR of 13.38% during the forecast period. Sustained growth of the market is propelled by the rising integration of expanded autoantibody panels into routine clinical pathways for earlier identification of systemic autoimmune disorders.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 42.78%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 15.38%.
- Based on Product, reagents & assay kits dominated the market with a revenue share of 47.81%.
- Based on Technique, the Immunofluorescence Assay segment dominated the market with a 53.45% share in 2025.
- By Application, the Rheumatoid Arthritis segment held the dominant share of 34.72% in 2025.
- By End Use, the Hospitals segment dominated with a 42.34% share in 2025.
- The U.S. dominates the global antinuclear antibody test market, valued at USD 718.92 million in 2024 and reaching USD 812.02 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
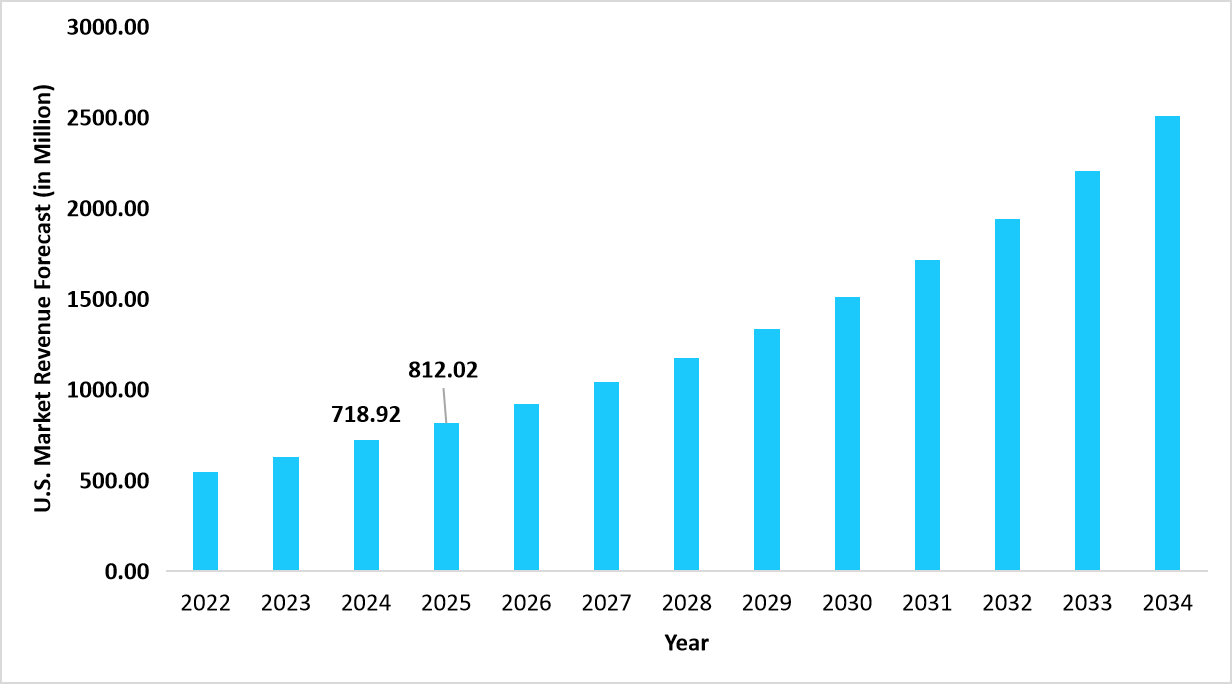
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.10 billion
- 2034 Projected Market Size: USD 6.48 billion
- CAGR (2025 to 2034): 13.38%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The antinuclear antibody test market encompasses diagnostic products and platforms used to detect autoantibodies associated with connective tissue and systemic autoimmune disorders, covering a wide portfolio that includes reagents and assay kits, automated systems, and software and services that support analytical workflow management. The market spans core techniques such as ELISA, immunofluorescence assay, and multiplex assay formats that enable screening, confirmation, and extended antibody profiling across diverse clinical conditions, including rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, scleroderma, and other related diseases. Demand is shaped by expanding use of these tests across hospitals, clinical laboratories, physician office laboratories, and additional healthcare settings where clinicians depend on structured autoantibody evaluation to guide diagnosis, monitor disease progression, and support personalized treatment planning across autoimmune care pathways.
Latest Market Trends
Transition Toward Pattern Standardization Through AI-supported Fluorescence Interpretation
A rising trend is the shift toward AI-supported interpretation modules that classify fluorescence patterns with higher consistency across large testing networks. Reference centers are increasingly integrating digital readers that convert visual patterns into quantitative profiles, creating a new framework for pattern interpretation that reduces reading variability across laboratories.
Expansion of Composite Autoantibody Panels For Early Immune Pathway Mapping
The major trend is the growing use of multiplex antibody sets designed to map immune pathway disturbances in earlier stages of autoimmune conditions. These composite panels extend beyond traditional markers and support broader clinical evaluation strategies across hospitals and specialty clinics.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.10 Billion |
| Estimated 2026 Value | USD 2.37 Billion |
| Projected 2034 Value | USD 6.48 Billion |
| CAGR (2026-2034) | 13.38% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., EUROIMMUN, Werfen, ZEUS Scientific, Inc. |
to learn more about this report Download Free Sample Report
Market Driver
Growing Adoption of Extended Autoimmune Profiling In Multidisciplinary Care Pathways
A key driver for the market is the rising incorporation of extended antibody profiling protocols across multidisciplinary care programs in rheumatology, endocrinology, and dermatology. As clinicians increase requests for broader immune system evaluation, laboratories adopt platforms capable of processing large antibody panels within integrated workflows.
Market Restraint
Variation In Pattern Reading Proficiency Across Laboratory Tiers Limits Uniform Interpretation
A restraint arises from variation in reader expertise across primary, secondary, and tertiary laboratory tiers. In regions where access to trained immunology personnel is uneven, pattern interpretation inconsistencies reduce testing uniformity, which slows wider adoption of advanced platforms across decentralized facilities.
Market Opportunity
Growth Of Decentralized Digital Immunology Hubs Supporting Large Population Screening Programs
An emerging opportunity is the establishment of digital immunology hubs across regions, expanding screening for autoimmune disorders. These hubs support large sample processing volumes through connected analyzers and centralized review centers, encouraging hospitals and diagnostic chains to expand procurement of high-throughput systems that fit into coordinated national screening models.
Regional Analysis
North America maintained a leading share of 42.78% in the antinuclear antibody test market in 2025, supported by expansions in autoimmune disease testing programs across large clinical laboratory networks in the U.S. and Canada. The region saw wider deployment of automated IFA and ELISA platforms as high-volume laboratories increased investments in connected immunology antinuclear antibody test analyser systems that streamlined testing workflows for rheumatology and primary care diagnostics.
In the U.S., market growth is shaped by the rise of advanced autoimmune reference centers that expanded assay menus covering extended antinuclear antibody TEST reflex panels. Continued funding for immunology research encouraged the commercial launch of specialized antigen profiles used for early interpretation of systemic autoimmune disorders across academic hospital networks and integrated diagnostic chains.
Asia Pacific Market Insights
Asia Pacific recorded the strongest growth pace of 15.38% during the forecast period, driven by expanding autoimmune testing capabilities within national health programs across China, India, Japan, and Southeast Asia. Rising adoption of high-throughput immunoassay systems in regional hospitals supported wider use of antinuclear antibody test screening as part of rheumatology evaluation pathways.
China showed elevated momentum as provincial laboratories incorporated multiplex autoantibody platforms that increased testing capacity for large patient populations. New laboratory expansions across urban centers encouraged broader use of antinuclear antibody test detection technologies for connective tissue disorder assessment.
Regional Market share (%) in 2025
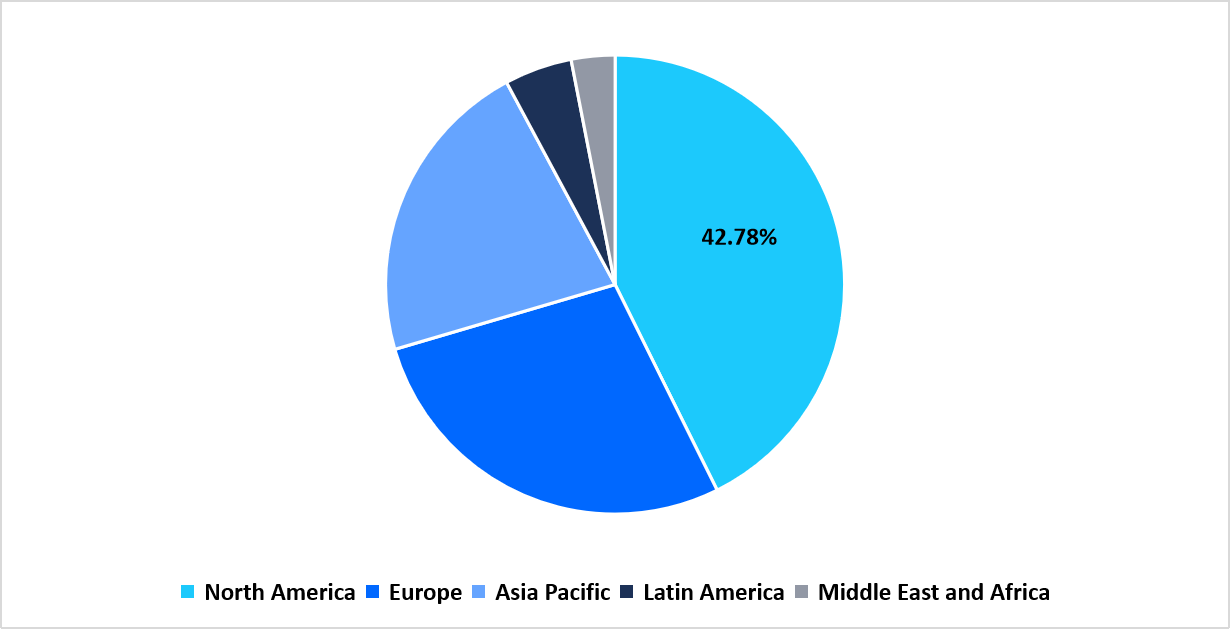
Source: Straits Research
Europe Market Insights
Europe observed steady advancement in antinuclear antibody test testing driven by regional focus on harmonized autoimmune diagnostic procedures across public health institutions. Standardized laboratory guidelines promoted the adoption of automated IFA readers and integrated platforms within national rheumatology networks.
In France, market progression is supported by the modernization of immunology departments, where hospitals transitioned from older fluorescence microscopes to digital systems capable of higher throughput. Expanded procurement programs strengthened access to updated antinuclear antibody test panels across metropolitan centers.
Latin America Market Insights
Latin America experienced growing adoption of antinuclear antibody testing as healthcare systems incorporated expanded autoimmune diagnostic services across tertiary hospitals and private laboratories. Broader availability of automated ELISA antinuclear antibody test analyzers supported market expansion in large urban regions.
Brazil recorded notable movement with new immunology laboratories established within public health networks, increasing domestic access to standardized antinuclear antibody test screening and follow-up testing. Enhanced diagnostic infrastructure supported higher testing volumes across major cities.
Middle East and Africa Market Insights
The Middle East and Africa region observed rising activity in antinuclear antibody test adoption, supported by national programs that strengthened autoimmune disease evaluation capacity across public hospitals and specialty clinics. New diagnostic centers introduced advanced immunology antinuclear antibody test analyzers to expand screening coverage within rheumatology care pathways.
In the United Arab Emirates, growth stemmed from new immunodiagnostic laboratories established within major healthcare development zones, where facilities added automated antinuclear antibody test systems to support rising test volumes across both public and private providers.
Product Insights
The Reagents and Assay Kits segment dominated the market in 2025 with a 47.81% share. This position resulted from rising test volumes across hospitals and clinical laboratories where assay kits supported routine screening and reflex testing workflows. Expanded availability of antigen panels across autoimmune diagnostics further strengthened demand for this segment.
The Systems segment recorded the fastest growth during the forecast period. Growth was driven by increasing installation of automated platforms that supported higher throughput testing and reduced manual interpretation variability across busy immunology laboratories. Advancements in integrated reader technologies encouraged broader placement of system-based solutions in both public and private testing facilities.
Technique Insights
The Immunofluorescence Assay segment dominated the market with a 53.45% share in 2025. Its leadership was supported by wide use of HEp2 substrate-based pattern evaluation across reference laboratories and specialized rheumatology centers. Continued preference for fluorescence-based visual patterns sustained strong adoption across established diagnostic networks.
The ELISA segment grew at the fastest pace during the forecast period. Growth emerged from expanding use of ELISA platforms in laboratories upgrading their autoimmune diagnostics workflows, where consistent throughput and ease of automation supported higher test processing volumes.
Application Insights
The Rheumatoid Arthritis segment held the dominant share of 34.72% in 2025. Its position stemmed from increasing testing requests across outpatient and inpatient rheumatology services, where laboratories processed larger volumes of samples tied to early evaluation of joint-related autoimmune conditions.
The Systemic Lupus Erythematosus segment represented the fastest-growing application. This rise was influenced by expanding utilization of extended antibody panels across tertiary care centers, supporting detailed assessment of multisystem autoimmune presentations.
End Use Insights
The Hospitals segment dominated with a 42.34% share in 2025 due to expanded inpatient and outpatient testing volumes. Rising establishment of rheumatology units within large medical centers supported higher demand for laboratory-based autoimmune diagnostics.
The Clinical Laboratories segment recorded the fastest growth during the forecast period as independent diagnostic chains broadened their immunology testing menus and adopted automated platforms to support increasing sample inflow from outpatient clinics and physician offices.
By End Use Market Share (%), 2025
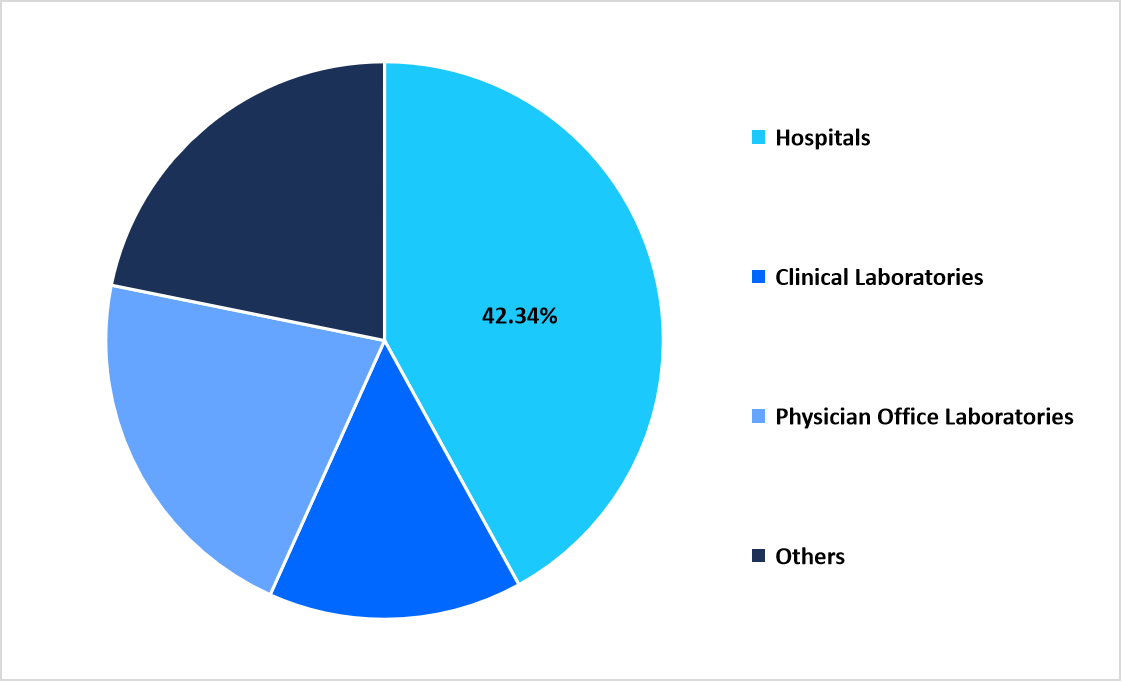
Source: Straits Research
Competitive Landscape
The global antinuclear antibody test market features a moderately consolidated competitive environment, shaped by established diagnostic companies and specialized autoimmune assay developers that focus on ELISA, immunofluorescence assays IFA, multiplex platforms, and automated antinuclear antibody test analyzers. Leading players compete through proprietary antigen panels, automation-focused assay processing, expanded autoimmune menus, and strategic geographic expansion to strengthen their presence across clinical laboratories and hospital-based testing settings.
EUROIMMUN: An Emerging Market player
- EUROIMMUN holds a prominent position through its wide ANTINUCLEAR ANTIBODY TEST testing portfolio that includes IFA based HEp2 cell substrates and automated platforms such as EUROLabWorkstation and EUROPattern. The company continues to broaden its assay menu and expand system integration across laboratories, giving it a strong role in shaping antinuclear antibody test standardization and workflow enhancement across global markets.
List of Key and Emerging Players in Antinuclear Antibody Test Market
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc.
- EUROIMMUN
- Werfen
- ZEUS Scientific, Inc.
- Antibodies Incorporated
- Trinity Biotech plc
- Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- Corewell Health
- Abcam Limited
- GROUP GmbH
- Immuno Concepts NA Ltd
- Medical & Biological Laboratories Co., Ltd.
- Others
Strategic Initiatives
- October 2025: Tellgen invested in CytoCares to expand its autoimmune diagnostics capabilities and advanced its “Diagnosis-Therapy-Monitoring” model. The company’s CAP-recognized flow fluorescence platform detected 46 autoantibody targets, including a 16-item ANTINUCLEAR ANTIBODY TEST panel, and aimed to improve early detection, disease stratification, and patient monitoring while accelerating integrated diagnostic-therapy solutions in the growing autoimmune disease landscape.
- February 2025: Hipro Biotechnology Co., Ltd. (China) showcased cutting-edge diagnostic innovations at MedLab Middle East 2025, including the Fluorescence Immunoassay antinuclear antibody test analyzer (PalmF). However, infrastructure gaps, unequal laboratory capabilities, and lower overall adoption compared to leading Western markets limited the pace and penetration of these technologies. As a result, even though innovation remained essential and continued developing, it was still considered at a medium level rather than a high one.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.10 Billion |
| Market Size in 2026 | USD 2.37 Billion |
| Market Size in 2034 | USD 6.48 Billion |
| CAGR | 13.38% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Technique, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Antinuclear Antibody Test Market Segments
By Product
- Reagents & Assay Kits
- Systems
- Software & Services
By Technique
- ELISA
- Immunofluorescence Assay
- Multiplex Assay
By Application
- Rheumatoid Arthritis
- Systemic Lupus Erythematosus
- Sjogren’s Syndrome
- Scleroderma
- Other Diseases
By End Use
- Hospitals
- Clinical Laboratories
- Physician Office Laboratories
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































