Artemisinic Acid Market Size, Share & Trends Analysis Report By Type (Total Synthesis, Semi synthesis), By Application (Antimalarial Injections, Antimalarial Tablets) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Artemisinic Acid Market Overview
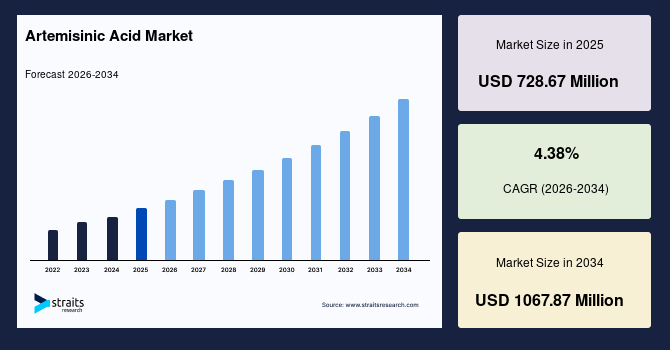
The global artemisinic acid market size is estimated at USD 728.67 million in 2025 and is projected to reach USD 1067.87 million in 2034, growing at a CAGR of 4.38% during the forecast period. The remarkable growth of the market is due to the rising transition toward controlled synthesis pathways that support consistent production of intermediates used in artemisinin-based therapies.
Key Market Trends & Insights
- Middle East and Africa held a dominant share of the global market, accounting for 37.24% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 6.38%.
- Based on type, semi semi-synthesis segment is anticipated to register the fastest CAGR of 5.12% during the forecast period.
- Based on application, antimalarial tablets are anticipated to register the fastest CAGR of 5.35% during the forecast period.
- South Africa dominates the global artemisinin acid market, valued at USD 118.39 million in 2024 and reaching USD 123.06 million in 2025.
Graph: South Africa Market Revenue Forecast (2022 – 2034)
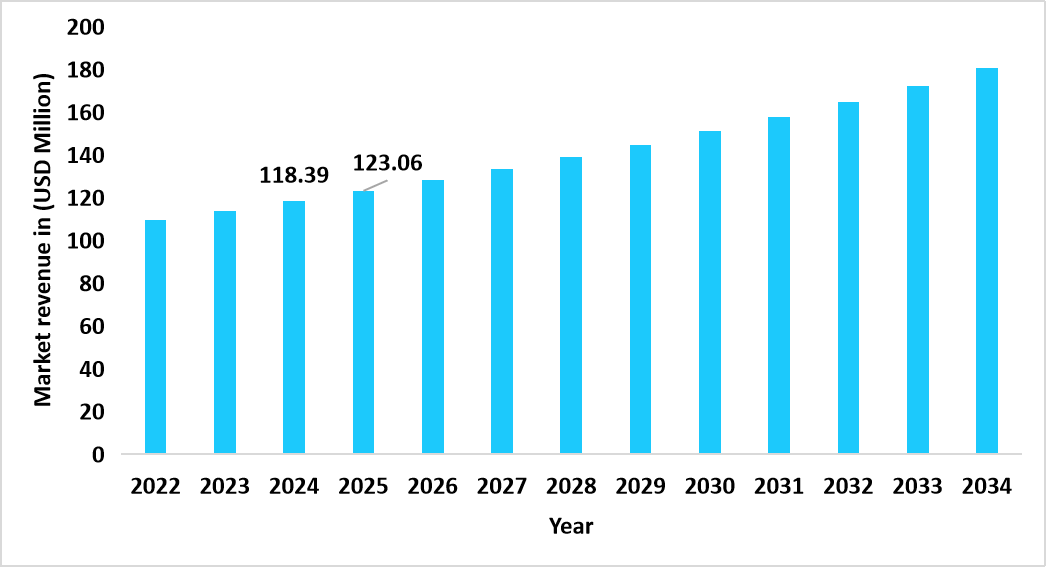
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 728.67 million
- 2034 Projected Market Size: USD 1067.87 million
- CAGR (2025 to 2034): 4.38%
- Dominating Region: Middle East and Africa
- Fastest-Growing Region: Asia Pacific
The artemisinic acid market encompasses the production, processing, and commercial supply of artemisinic acid, a key biochemical precursor used for synthesizing artemisinin and its derivatives that serve as the foundation of global antimalarial therapy. The market includes two core production pathways: total synthesis, which relies on controlled chemical processes, and semi-synthesis, which integrates biological or plant-derived intermediates with downstream chemical conversion. Artemisinic acid supports a wide range of applications within antimalarial treatment protocols, with demand driven by its role in the manufacture of antimalarial injections used for severe malaria and antimalarial tablets formulated for widespread treatment of uncomplicated malaria. As global health programs continue to prioritize artemisinin-based therapies, the market for artemisinic acid remains integral to the stability and growth of the antimalarial pharmaceutical landscape.
Latest Market Trends
Shift from Plant-Dependent Extraction to Precision-Controlled Fermentation Pathways
The artemisinic acid market is experiencing a steady shift from cultivation-based extraction of Artemisia annua to fermentation pathways that generate consistent precursor outputs through engineered microbial processes. Earlier systems relied on variable agricultural cycles with fluctuating plant yields, but new production models utilize controlled fermentation to streamline intermediate availability. This shift reshapes long-term planning for companies aiming to stabilize input supply for artemisinin conversion.
Shift from Single-Source Supply Chains to Distributed Manufacturing Networks Across Multiple Regions
Manufacturers are transitioning from centralized manufacturing hubs to distributed networks that place fermentation and processing units closer to end-use pharmaceutical markets. Previous models concentrated artemisinic acid output in select countries, creating exposure to logistics delays and export constraints. New strategies distribute production across varied geographies, supporting faster delivery timelines and improved alignment with local antimalarial formulation facilities.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 728.67 Million |
| Estimated 2026 Value | USD 760.59 Million |
| Projected 2034 Value | USD 1067.87 Million |
| CAGR (2026-2034) | 4.38% |
| Dominant Region | Middle East and Africa |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Sanofi, PATH, KPC Pharmaceuticals, Inc, Guilin Pharmaceutical Co., Ltd, Calyx Chemicals and Pharmaceuticals Ltd. |
to learn more about this report Download Free Sample Report
Artemisinic Acid Market Driver
Growing Interest in High-Purity Intermediates for Advanced Artemisinin Derivative Formulation
Demand for high-purity artemisinic acid continues to expand as manufacturers refine conversion processes for artesunate, artemether, and other derivatives used in therapy protocols. The focus on improved intermediate quality supports wider adoption of upgraded synthesis pathways and encourages investment in equipment designed for controlled precursor output across fermentation and chemical processing settings.
Market Restraint
Fluctuations in Artemisia annua Crop Economics Affecting Feedstock Planning for Hybrid Producers
Manufacturers that combine plant extraction with semi-synthetic processes face ongoing uncertainty tied to shifting crop pricing, regional cultivation setbacks, and variable harvest cycles. These fluctuations complicate feedstock forecasting for companies that integrate both agricultural inputs and fermentation intermediates, slowing expansion plans for hybrid supply models.
Market Opportunity
Rising Collaboration Between Biotechnology Firms and Public Health Agencies to Strengthen Antimalarial Supply Programs
Growing partnerships between biotech developers, global health agencies, and regional procurement bodies are creating fresh avenues for expanding artemisinin acid availability across high-burden malaria regions. These collaborations support new contract manufacturing arrangements, technology-transfer initiatives, and coordinated planning for downstream API distribution, offering substantial growth potential for producers seeking long-term stability.
Regional Analysis
Middle East and Africa lead the global artemisinin acid market with a 37.24% share due to high malaria prevalence, long-standing integration of artemisinin-based therapies in national treatment guidelines, and expanding procurement frameworks for both plant-derived and semi-synthetic intermediates. Regional ministries coordinate cross-country supply networks to ensure adequate API availability. Growth in local pharmaceutical manufacturing supports broader adoption of artemisinin-based combination therapies across the continent.
The South Africa artemisinic acid market advances through expansion of public-sector antimalarial programs, increased participation in clinical research on artemisinin-based therapies, and strengthening of distribution systems supplying treatment centers. Laboratories in major provinces develop improved screening systems for malaria diagnosis, supporting ongoing demand for artemisinin-derived APIs in both public and private healthcare settings.
Asia Pacific Market Insights
Asia Pacific records the fastest growth of 6.38% due to high malaria incidence in various tropical regions, rising investments in plant-derived artemisinin extraction, and increasing regional adoption of semi-synthetic production methods across China, India, Vietnam, and Thailand. Local governments reinforce procurement plans for artemisinin-based therapies, while cultivation of Artemisia annua expands across diverse agro-climatic zones. Pharmaceutical companies broaden participation in active-ingredient processing to strengthen downstream supply availability.
The China artemisinic acid market develops through expansion of Artemisia cultivation acreage, increased domestic fermentation capacity, and wider involvement of university-affiliated research groups in strain-optimization studies. Provincial authorities encourage industrial partnerships that support consistent production of intermediates required for artemisinin conversion within national malaria-control frameworks.
Pie Chart: Regional Market Share, 2025
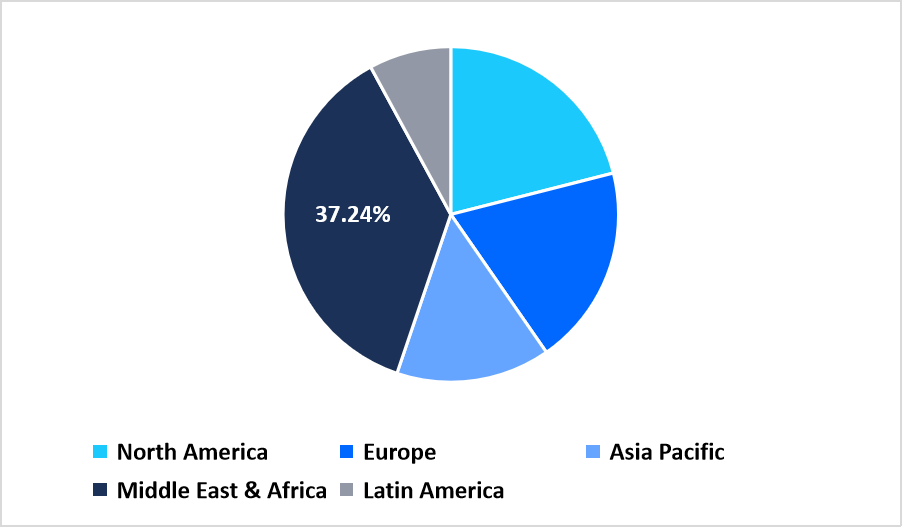
Source: Straits Research
North America Market Insights
North America maintains a steady position in the artemisinin acid market due to established pharmaceutical production systems, sustained adoption of semi-synthetic artemisinin pathways, and continued partnerships between research laboratories and malaria-focused health programs. Collaboration between academic institutes and fermentation-technology developers supports refinement of upstream biochemical processes. Regulatory agencies coordinate with drug manufacturers to maintain consistency in API supply chains used for artemisinin-based therapies.
The U.S. artemisinin acid market progresses through expansion of precision-fermentation projects, broader engagement of biotechnology firms in microbial engineering, and continued integration of artemisinin-derived APIs into antimalarial drug development programs for global distribution. Universities advance metabolic-pathway research, while manufacturers pursue additional scale-up cycles for yeast-derived intermediates.
Europe Market Insights
Europe advances its position through harmonized regulatory pathways, cross-border collaboration on malaria drug supply, and continued evaluation of plant-based and fermentation-based production techniques. Research agencies examine sustainability metrics for cultivation and synthetic processes, while manufacturers assess procurement channels for intermediates. Growth in travel-associated malaria cases encourages additional focus on stable sourcing of artemisinin-derived ingredients.
The UK artemisinin acid market evolves through joint projects between academic laboratories and API suppliers, refinement of extraction methods within niche production facilities, and introduction of updated workflows for artemisinin conversion. Institutional reviews support assessment of quality parameters, while national programs strengthen access to malaria treatment materials for imported cases.
Latin America Market Insights
Latin America maintains a growing role due to persistent malaria transmission in parts of the Amazon region, government-supported treatment programs, and renewed interest in hybrid extraction-fermentation supply models. Cross-country cooperation enhances access to artemisinin-derived substances, while pharmaceutical producers evaluate opportunities for domestic API processing. Expansion of rural healthcare networks supports the availability of artemisinin-based therapies in high-incidence zones.
The Argentine artemisinin acid market progresses through regional research efforts examining artemisinin conversion pathways, increased academic involvement in metabolic-engineering studies, and refinement of procurement plans within national malaria-response frameworks. Local biotech companies explore feasibility studies on semi-synthetic production methods to support future API supply stability.
Type Insights
Total Synthesis dominates the market, supported by expanding adoption of controlled chemical processes that allow producers to maintain consistent yields of artemisinic acid across large-scale manufacturing environments. Companies pursuing this route benefit from reduced dependence on agricultural cycles and enhanced management of precursor availability. Growing emphasis on standardized output reinforces the continued preference for total synthesis across pharmaceutical supply networks engaged in artemisinin API production.
Semi-synthesis records the fastest growth at 5.12%, driven by rising investments in fermentation-based intermediate generation and wider interest in hybrid production models that combine biological and chemical methods. Expansion of Artemisia annua cultivation in select regions contributes to increased precursor availability. Research groups continue to refine conversion pathways, bringing additional attention to semi-synthetic routes as producers explore new methods to support long-term API stability.
Application Insights
Antimalarial Injections dominate the market, supported by broad utilization of injectable artesunate across treatment protocols for severe malaria in hospital and emergency settings. Demand for high-purity artemisinin-derived intermediates continues to rise as global health agencies reinforce treatment standards for acute cases. Strengthening of procurement channels across malaria-endemic countries maintains consistent reliance on artemisinin acid for the formulation of parenteral therapies.
Antimalarial Tablets record the fastest growth at 5.35%, driven by expanding adoption of oral artemisinin-based combination therapies across outpatient care systems. Broader distribution initiatives across rural and semi-urban regions support increased tablet usage for uncomplicated malaria. Growing participation of regional manufacturers in fixed-dose combination production continues to contribute to the rapid growth of this segment, solidifying its position within the downstream market for artemisinic acid derivatives.
Competitive Landscape
The global artemisinic acid market is moderately fragmented, consisting of established pharmaceutical manufacturers, biotechnology developers, fermentation-based producers, and regional suppliers engaged in the extraction, synthesis, and downstream conversion of artemisinic acid–derived compounds and artemisinin-based APIs.
Amyris, Inc.: An emerging market player
Amyris, Inc., a biotechnology company known for its fermentation-based production platforms, has been a central contributor to the global supply of bio-engineered artemisinic acid. The company’s microbial synthesis technology supports consistent-scale production of artemisinic acid, a key precursor for semi-synthetic artemisinin used in antimalarial therapies. Amyris continues to align its manufacturing capabilities with global demand for intermediates used in artemisinin-based APIs.
List of Key and Emerging Players in Artemisinic Acid Market
- Sanofi
- PATH
- KPC Pharmaceuticals, Inc
- Guilin Pharmaceutical Co., Ltd
- Calyx Chemicals and Pharmaceuticals Ltd.
- Novartis AG
- Ipca Laboratories Ltd.
- Cipla Inc.
- CO, LTD
- AdvaCare Pharma
- Ajanta Pharma Ltd.
- Mylan N.V.
- Strides Pharma Science Limited
- Zydus Cadila
- Amyris, Inc.
- Others
Strategic Initiatives
- May 2025: Amyris, Inc. took full ownership of its Brazilian industrial fermentation plant in Barra Bonita by acquiring the remaining 31% stake from its partner. It also committed to complete a fourth precision-fermentation line at that facility.
- May 2025: Sanofi committed to investing at least US$20 billion in the United States through 2030, directing funds toward expanded research & development and enhanced domestic manufacturing capacity.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 728.67 Million |
| Market Size in 2026 | USD 760.59 Million |
| Market Size in 2034 | USD 1067.87 Million |
| CAGR | 4.38% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Artemisinic Acid Market Segments
By Type
- Total Synthesis
- Semi synthesis
By Application
- Antimalarial Injections
- Antimalarial Tablets
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































