Asthma Therapeutics Market Size, Share & Trends Analysis Report By Drug Class (Anti Inflammatory, Bronchodilators, Combination Therapy), By Product (Inhalers, Dry Powder, Metered Dose, Soft Mist, Nebulizers, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Asthma Therapeutics Market Overview
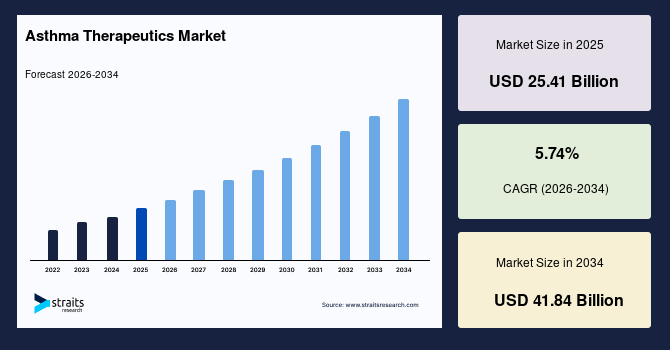
The global asthma therapeutics market size is valued at USD 25.41 billion in 2025 and is estimated to reach USD 41.84 billion by 2034, growing at a CAGR of 5.74% during the forecast period. The consistent market growth is fuelled by the Growing utilization of genomic biomarkers, enabling personalized asthma treatment optimization.
Key Market Trends & Insights
- North America held a dominant revenue share of the global market, accounting for 47.31% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 6.97%.
- Based on drug class, the anti-inflammatory segment held the highest market share of 62.83% in 2025.
- By product, the nebulizer segment is expected to register the fastest CAGR growth of 6.37%.
- The U.S. dominates the market, valued at USD 10.29 billion in 2024 and reaching USD 10.83 billion in 2025.
Table: U.S. Asthma Therapeutics Market Size (USD Million)
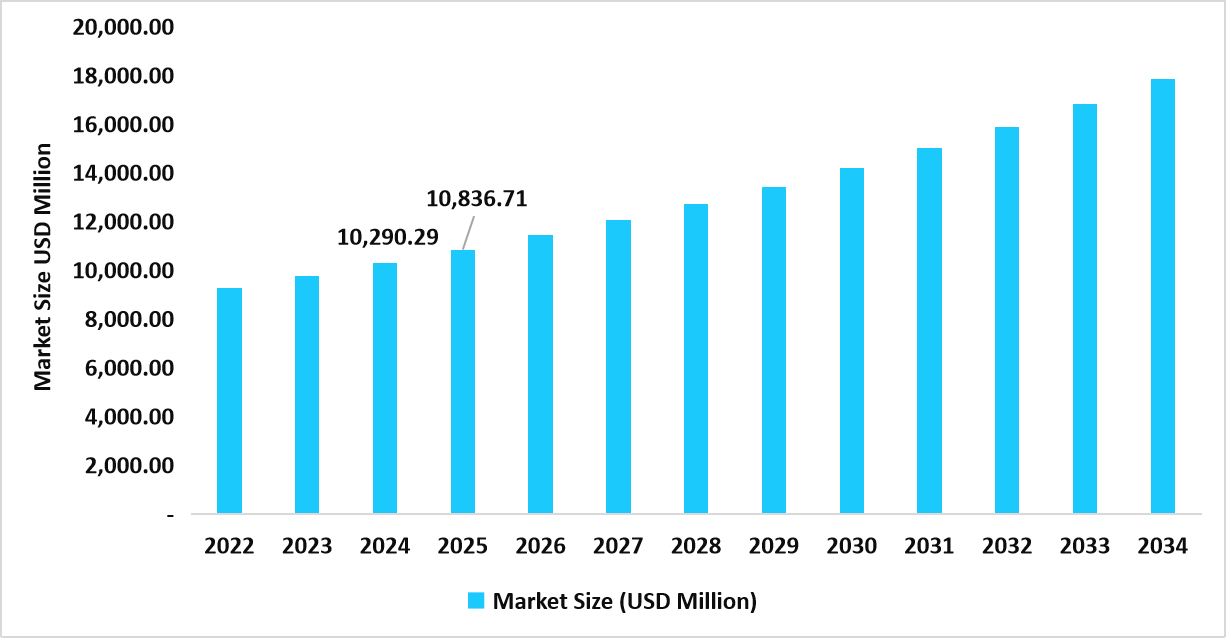
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 25.41 billion
- 2034 Projected Market Size: USD 41.84 billion
- CAGR (2026-2034): 5.74%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The asthma therapeutics market comprises a comprehensive range of pharmacological solutions designed to manage and control asthma symptoms. The market includes drug classes such as anti-inflammatory agents, bronchodilators, and combination therapies, delivered through products like inhalers, including dry powder, metered dose, and soft mist, along with nebulizers and other supportive devices.
Latest Market Trends
Growing Adoption Of Digital Inhalers With Integrated Adherence Analytics
The rising use of digital inhalers equipped with embedded sensors that track real-time usage patterns and inhalation technique is a key trend for market growth. These devices transmit adherence data to clinicians via connected platforms, enabling personalized treatment adjustments and early intervention for uncontrolled symptoms. This technology is reshaping asthma management by improving medication compliance, reducing exacerbations, and supporting data-driven clinical decision-making.
Rising Use of Pharmacogenomic Profiling for Personalized Asthma Drug Selection
A key trend in the asthma therapeutics market is the integration of pharmacogenomic profiling to tailor drug selection based on a patient’s genetic response to inhaled corticosteroids, bronchodilators, or biologics. This approach enables clinicians to identify patients with steroid-resistant asthma, optimize biologic eligibility, and reduce trial-and-error prescribing. As precision medicine expands, genetic markers such as IL-4Rα variants and β2-adrenergic receptor polymorphisms are increasingly influencing individualized treatment pathways.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 25.41 Billion |
| Estimated 2026 Value | USD 26.77 Billion |
| Projected 2034 Value | USD 41.84 Billion |
| CAGR (2026-2034) | 5.74% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | AstraZeneca, Boehringer Ingelheim International GmbH, Chiesi Farmaceutici S.p.A., Cipla Limited, Hoffmann-La Roche Ltd. |
to learn more about this report Download Free Sample Report
Market Driver
Increasing Adoption Of Biomarker-guided Therapy Optimization
The growing use of biomarker-guided treatment pathways to improve therapeutic precision and outcomes. Healthcare providers are increasingly incorporating biomarkers such as blood eosinophil count, FeNO levels, and periostin expression to identify patient phenotypes and match them with the most effective biologic or anti-inflammatory therapy. This targeted approach reduces unnecessary medication use, enhances response predictability, and supports the wider adoption of advanced, personalized asthma treatment strategies.
Market Restraint
Limited Access To Biologic Testing Infrastructure
The limited availability of diagnostic infrastructure is required to evaluate eligibility for advanced biologic therapies. Biomarker assessments such as eosinophil profiling, FeNO testing, and IgE quantification are essential for determining suitability; however, many clinics in emerging regions lack these specialized testing capabilities. This gap restricts patient access to targeted treatments, delays optimized therapy decisions, and ultimately limits the broader adoption of precision-based asthma therapeutics in resource-limited healthcare systems.
Market Opportunity
Growing Application Of Microbiome-targeted Therapies
The development of microbiome-modulating treatments aimed at restoring healthy airway microbial balance presents a major opportunity for market growth. Research advancements indicate that specific bacterial compositions influence asthma severity, treatment responsiveness, and inflammation pathways. This has fueled interest in probiotics, live biotherapeutics, and inhaled microbiome-based interventions as complementary or standalone therapies. As clinical validation progresses, microbiome-targeted solutions present a new therapeutic frontier with strong commercialization potential in precision respiratory medicine.
Regional Analysis
North America dominated the market in 2025, accounting for 47.31% market share. Growth in the region is supported by the region’s rapid expansion of specialised severe asthma clinics equipped to administer biologics and perform advanced phenotyping tests. These dedicated centers streamline eligibility assessment, optimize treatment pathways, and increase adoption of novel therapeutics, notably boosting regional demand for advanced asthma management solutions.
The U.S. asthma therapeutics market is experiencing unique growth driven by the expansion of employer-supported respiratory wellness programs that include subsidized access to advanced asthma therapeutics and digital monitoring tools. These workplace initiatives aim to reduce absenteeism and improve chronic disease management, indirectly increasing nationwide adoption of modern asthma treatments and connected care technologies.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 6.97% from 2026-2034. This growth is attributed to the rising deployment of mobile health clinics offering inhaler and nebuliser therapies in rural and semi-urban areas. This approach improves accessibility for underserved populations, enhances early asthma management, and expands the regional market by reaching patients beyond traditional hospital and urban healthcare settings.
Australian asthma therapeutics market is experiencing strong growth during the forecast period. This expansion is attributed to the integration of remote Indigenous healthcare programs providing personalised inhaler and biologic therapies. These initiatives address high asthma prevalence in Aboriginal and Torres Strait Islander communities, improving treatment adherence, reducing hospitalisations, and expanding market demand for region-specific advanced asthma management solutions.
Regional Market share (%) in 2025
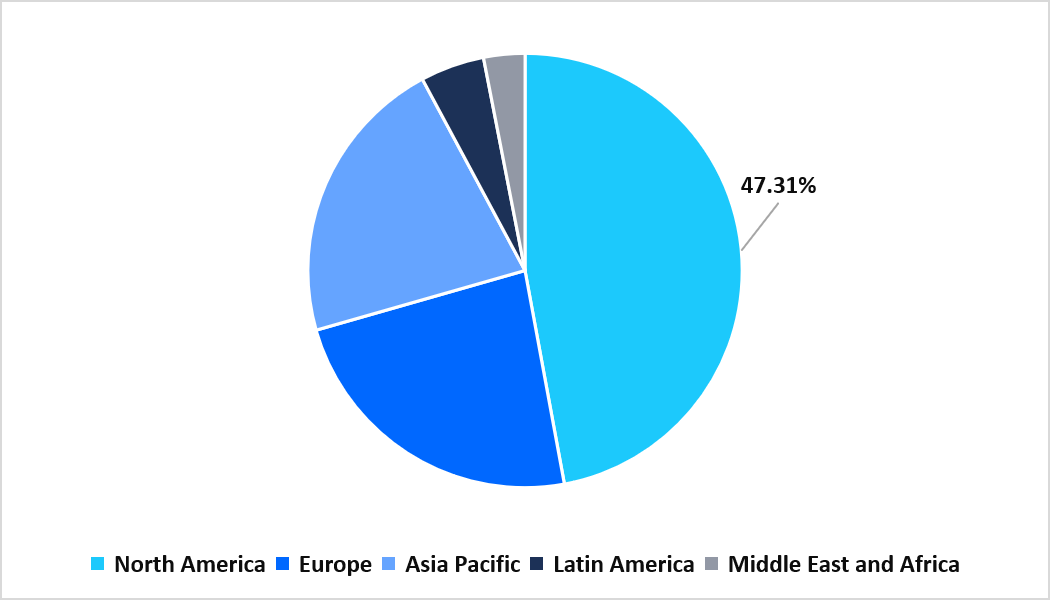
Source: Straits Research
Europe Market Insights
Europe is witnessing strong growth in the asthma therapeutics market, driven by the widespread adoption of inhaled biologics targeting severe eosinophilic asthma, supported by region-specific reimbursement policies and guideline endorsements from the European Respiratory Society, which accelerate clinical uptake and expand demand for advanced combination and biologic therapies.
The UK asthma therapeutics market is stimulated by the integration of National Health Service (NHS) digital asthma registries with prescription-linked adherence tracking. This system enables clinicians to monitor patient response to biologics and combination therapies in real time, improving treatment optimisation and increasing demand for advanced asthma therapeutics across the country.
Latin America Market Insights
The Latin American asthma therapeutics market is benefiting from the expansion of public-private partnerships to supply subsidized inhalers and nebulizers in underserved urban and rural areas. These initiatives improve treatment accessibility, increase early intervention for chronic asthma, and stimulate demand for both standard and advanced asthma therapeutics across the region.
The Brazilian asthma therapeutics market is propelled by the growing adoption of telemedicine initiatives combined with portable nebulizer distribution. These programs address limited healthcare access in remote rainforest communities, enabling early intervention and consistent treatment adherence, thereby increasing demand for advanced and portable asthma therapeutics.
Middle East and Africa Market Insights
The Middle East and Africa Asthma therapeutics market is being driven by rising urban air pollution and densely populated cities, which have increased asthma prevalence to approximately 10.61% among children and adolescents. This environmental risk is boosting demand for inhalers, combination therapies, and maintenance treatments across the region.
The Saudi Arabia asthma therapeutics market is benefiting from the implementation and frequent updates of the Saudi Initiative for Asthma (SINA). These evidence-based national guidelines standardise diagnosis and treatment protocols, encourage the use of biologics and modern inhaler therapies, and increase clinician confidence, thereby accelerating uptake of advanced asthma therapeutics across the Kingdom.
Drug Class Insights
The anti-inflammatory segment dominated the market with a revenue share of 62.83% in 2025. This dominance is attributed to growing clinical adoption of airway-targeted nanoformulations that enhance corticosteroid penetration in distal bronchi. These ultra-fine particle technologies improve drug deposition, reduce systemic exposure, and deliver superior inflammation control, strengthening demand for advanced anti-inflammatory therapies.
The combination therapy segment is projected to grow at the fastest CAGR of 6.21% during 2026-2034, owing to the emergence of chronotherapy-based inhaler formulations designed to synchronize dual drug release with circadian inflammation cycles. This time, aligned therapies enhance nocturnal symptom control, improve overall treatment responsiveness, and drive stronger demand for advanced combination regimens.
By Technology Market Share (%), 2025
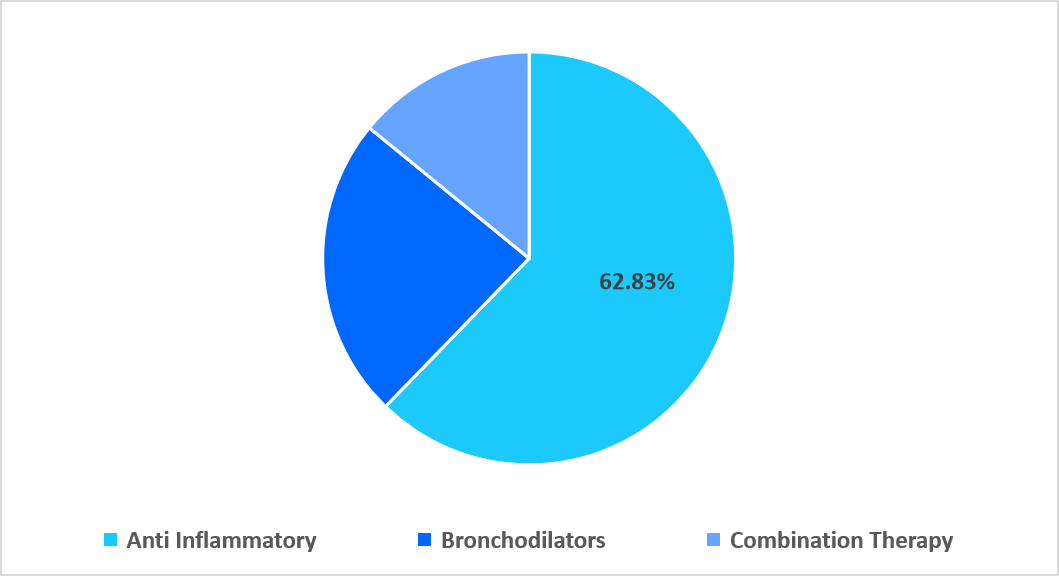
Source: Straits Research
Product Insights
The inhalers segment dominated the market in 2025. This dominance is augmented by the rising integration of dose accuracy microvalve technology within modern inhaler devices. These precision-engineered microvalves deliver highly consistent particle dispersion with each actuation, notably improving therapeutic reliability.
The nebulizers segment is anticipated to register the fastest CAGR of 6.37% during 2026-2034. This strong growth is driven by the emergence of piezoelectric mesh systems designed for nighttime pediatric and geriatric use. These near-silent nebulizers improve treatment adherence among noise-sensitive patients, especially during nocturnal symptom spikes, driving stronger adoption of next-generation home-based nebulization devices.
Competitive Landscape
The global asthma therapeutics market is highly consolidated, with major players such as GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, and Merck & Co. dominating through extensive inhaler and biologic portfolios. These companies focus on launching advanced therapies, expanding regional presence, and investing in R&D. Simultaneously, regional players like Teva and Cipla target cost-sensitive markets with generics, while emerging biotech firms leverage smart inhalers and digital monitoring to enhance patient-centric asthma management.
Areteia Therapeutics: An Emerging Market Player
Areteia Therapeutics is an emerging innovator in the asthma therapeutics market, focusing on developing novel oral treatments for eosinophilic and severe asthma. In 2025, the company secured USD 350 million in funding to advance its clinical pipeline. By offering convenient, non-invasive oral therapies as alternatives to traditional inhalers and biologics, Areteia is positioned to improve patient adherence, expand treatment options, and strengthen its presence in both developed and emerging markets.
List of Key and Emerging Players in Asthma Therapeutics Market
- AstraZeneca
- Boehringer Ingelheim International GmbH
- Chiesi Farmaceutici S.p.A.
- Cipla Limited
- Hoffmann-La Roche Ltd.
- GSK plc
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma
- Novartis AG
- Pfizer Inc.
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Covis Pharma Group
- Astellas Pharma Inc.
- Vertex Pharmaceuticals Incorporated
- Others
Strategic Initiatives
-
October 2025: AstraZeneca’s Tezspire received the U.S. FDA approval for chronic rhinosinusitis in the U.S.
- March 2025: The U.S. Food and Drug Administration (FDA) approved Amneal's albuterol sulfate inhalation aerosol for asthma and COPD patients.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 25.41 Billion |
| Market Size in 2026 | USD 26.77 Billion |
| Market Size in 2034 | USD 41.84 Billion |
| CAGR | 5.74% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Drug Class, By Product |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Asthma Therapeutics Market Segments
By Drug Class
- Anti Inflammatory
- Bronchodilators
- Combination Therapy
By Product
- Inhalers
- Dry Powder
- Metered Dose
- Soft Mist
- Nebulizers
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































