Automated Sample Preparation Technology Market Size, Share & Trends Analysis Report By Product & Service (Instruments, Consumables, Software & Services), By Application (Clinical Diagnostics, Genomics & Next-generation Sequencing, Proteomics & Metabolomics, Drug Discovery & Development, Others), By End Use (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Hospitals & Diagnostic Laboratories, Other) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Automated Sample Preparation Technology Market Overview
The global automated sample preparation technology market size is valued at USD 1.35 billion in 2025 and is estimated to reach USD 3.10 billion by 2034, growing at a CAGR of 9.68% during the forecast period. The consistent market growth is supported by the rising transition of laboratories toward high-throughput workflows that depend on standardized and automated sample processing.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 45.84% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 11.68%.
- Based on product & service, the Instruments segment held the highest revenue share of 32.63% in 2025.
- Based on application, the Clinical Diagnostics segment dominated the market with 34.56% in 2025.
- By end use, the Pharmaceutical and Biotechnology Companies segment held the largest market share of 41.24% in 2025.
- U.S. dominates the market, valued at USD 513.56 million in 2024 and reaching USD 561.07 million in 2025.
Table: U.S. Automated Sample Preparation Technology Market Size (USD Million)
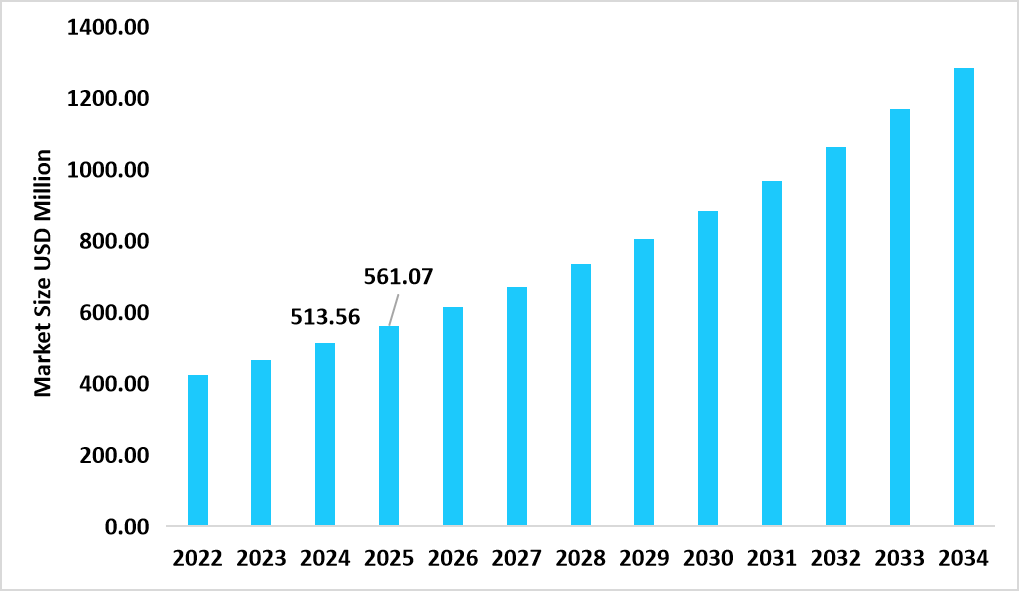
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.35 billion
- 2034 Projected Market Size: USD 3.10 billion
- CAGR (2026-2034): 9.68%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The automated sample preparation technology market encompasses systems, consumables, and digital tools designed to streamline and standardize the preparation of biological, chemical, and clinical samples across research, diagnostic, and biopharmaceutical environments. This market includes instruments that automate extraction, purification, and assay setup, consumables formatted for seamless integration with automated workflows, and software and services that coordinate instrument performance and data handling. Automated sample preparation supports applications across clinical diagnostics, genomics and next-generation sequencing, proteomics and metabolomics, drug discovery and development, and other laboratory activities that require consistent sample processing. End users include pharmaceutical and biotechnology companies, academic and research institutes, hospitals and diagnostic laboratories, and various specialized laboratories that utilize automation to improve throughput, accuracy, and workflow uniformity in molecular and biochemical studies.
Latest Market Trends
Growing Integration of AI-Guided Workflow Optimization Within Automated Sample Preparation Systems
A rising trend includes the adoption of AI-guided decision tools embedded into automation platforms to enhance workflow alignment and reduce operator-driven variability. These systems interpret instrument performance data, reagent usage patterns, and sample quality indicators to recommend adjustments that improve run consistency. Laboratories apply AI-driven optimization to streamline complex assays such as multiplex PCR setup, metagenomic extraction sequences, and multi-step purification processes. As automation environments expand, AI-enabled orchestration supports smoother coordination of sequential tasks, allowing laboratories to refine protocols with greater precision and improve processing continuity across diverse sample categories.
Increasing Shift Toward Microfluidic-Based Sample Preparation Formats
A parallel trend is the rising preference for microfluidic cartridges and chip-based sample preparation modules designed to condense extraction, purification, and reaction setup into compact channels. These formats reduce reagent volumes, minimize manual transfer steps, and create standardized fluid pathways suited for high-throughput research environments. Microfluidic systems are gaining traction in single-cell studies, point-of-care molecular workflows, and high-density screening platforms where controlled flow dynamics enhance sample uniformity. Adoption of these chips supports laboratory goals for reduced footprint, simplified operation, and streamlined integration with analytical devices.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.35 Billion |
| Estimated 2026 Value | USD 1.48 Billion |
| Projected 2034 Value | USD 3.10 Billion |
| CAGR (2026-2034) | 9.68% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | AutoGen, Inc., Zymo Research Corporation, Aurora Biomed Inc., Hamilton Company, Agilent Technologies, Inc. |
to learn more about this report Download Free Sample Report
Market Driver
Rapid Expansion of High-Throughput Molecular Testing Programs Across Research And Clinical Environments
Growing demand for large-volume processing in genetic screening, pathogen detection, and biomarker analysis accelerates the adoption of automated sample preparation systems. High-throughput programs frequently require consistent preparation of hundreds to thousands of samples, making automation essential for continuity of extraction quality, assay setup, and reagent application. Expansion of large cohort studies, population-based sequencing initiatives, and multi-site testing workflows encourages laboratories to invest in systems that maintain uniform processing speeds while reducing manual workloads.
Market Restraint
Limited Cross-Platform Compatibility Across Automated Instruments And Consumables
A restraint arises from interoperability gaps between instruments, software systems, and automation-compatible consumables. Variations in cartridge designs, pipette formats, fluid handling parameters, and communication interfaces can restrict seamless integration within mixed-platform laboratory environments. Laboratories operating multiple automation brands may encounter added validation tasks, workflow reconfiguration requirements, or software translation layers that slow adoption. These compatibility constraints create friction for teams aiming to scale multi-system workflows without added engineering adjustments.
Market Opportunity
Growing Investment In Decentralized Molecular Testing Centers Adopting Compact Automation Units
An expanding opportunity emerges as regional and satellite testing centers introduce compact automated systems to support their molecular workflows. These facilities require accessible platforms capable of handling extraction, assay setup, and sample normalization with minimal infrastructure. Compact automated units offer standardized workflows suitable for infectious disease testing, oncology panels, and targeted sequencing preparation in distributed diagnostic networks. As decentralized hubs expand across emerging regions and institutional laboratories, adoption of compact automated sample preparation platforms is positioned to accelerate, broadening market reach and enabling wider access to advanced molecular testing.
Regional Analysis
North America represents a leading region for automated sample preparation technologies with a 45.84% share driven by the broad adoption of automated extraction systems, liquid handling platforms, and workflow integration tools across genomics, molecular diagnostics, and drug discovery laboratories. The region benefits from sustained investment in laboratory modernization and close collaboration between automation vendors and clinical institutions, contributing to the rising deployment of sample processing instruments. Expansion of precision medicine and sequencing programs continues to strengthen demand for advanced sample preparation systems tailored to diverse throughput requirements.
In the U.S., growth is supported by the wider incorporation of automated extraction and purification technologies across clinical testing centers and research facilities. U.S. laboratories employ configurable automation platforms for nucleic acid workflows, assay preparation, and library construction, reinforcing the country’s leadership in automated sample handling adoption.
Asia Pacific Market Insights
Asia Pacific experiences fastest growth of 11.68% due to increased funding for genomics, proteomics, and molecular diagnostics capabilities. Regional laboratories expand automation use for extraction workflows, sample normalization, and high-throughput processing to support expanding research and clinical testing volume. Government-backed programs aimed at strengthening bioscience infrastructure continue to elevate uptake across academic and commercial laboratories.
In China, the market progresses with large-scale establishment of sequencing centers, automation-equipped laboratories, and technology parks. Chinese institutions integrate automated liquid handling systems and nucleic acid preparation platforms into multi-omics research, contributing to the steady expansion of sample preparation automation.
Regional Market share (%) in 2025
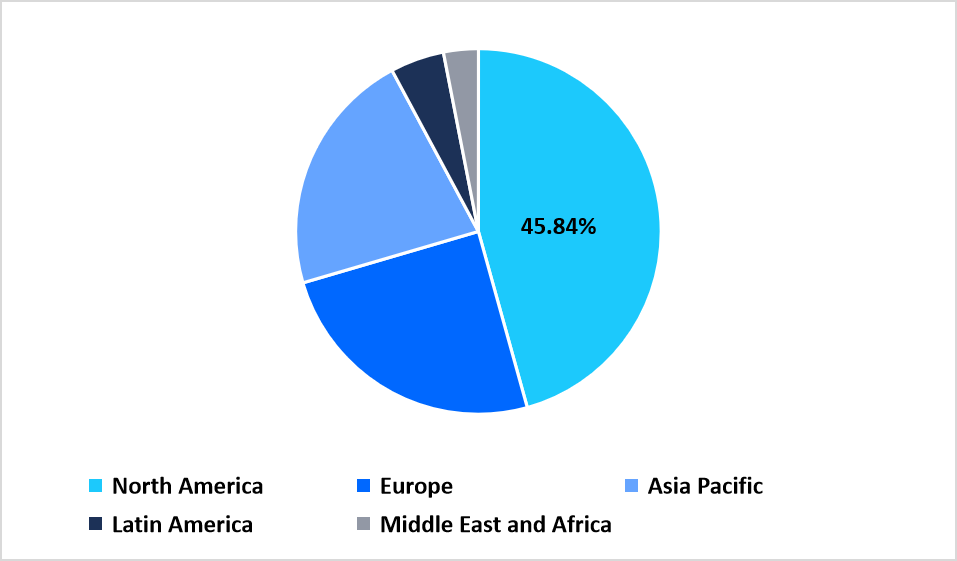
Source: Straits Research
Europe Market Insights
Europe maintains consistent adoption supported by interconnected scientific initiatives and laboratory networks that incorporate automated systems into clinical and research workflows. Regional facilities utilize automated platforms for sample extraction, assay setup, and workflow standardization across molecular testing environments. Growing investment in digital laboratory transformation further enhances adoption.
In Germany, development is driven by the widespread implementation of automation in translational research units and clinical molecular labs. German researchers incorporate automated extraction stations and pipetting systems into biomarker studies, sequencing pipelines, and diagnostic assay preparation.
Latin America Market Insights
Latin America experiences gradual advancement as universities, diagnostic centers, and public research institutions expand their molecular biology and genomics capabilities. Entry level automation tools gain adoption for sample extraction, workflow consistency, and basic sequencing preparation activities. Increased participation in international research initiatives supports the region’s technology uptake.
In Brazil, growth is reinforced by laboratory modernization efforts within academic institutions and emerging biotech clusters. Brazilian facilities adopt automated extraction units and liquid handling systems for molecular testing, research assays, and pilot-scale sequencing workflows.
Middle East and Africa Market Insights
The Middle East and Africa region advances as research universities, healthcare laboratories, and biotechnology hubs upgrade their molecular testing infrastructure. Standardized automated systems support workload expansion in nucleic acid extraction, assay preparation, and baseline molecular studies. Rising training programs in biosciences contribute to broader adoption.
In the United Arab Emirates, momentum accelerates with the development of new research campuses and clinical laboratories incorporating automation into daily workflows. UAE researchers use automated sample preparation platforms for genomic testing, molecular assays, and foundational research projects, strengthening the country’s position in regional laboratory automation.
Product & Service Insights
Instruments dominated the category with 32.63%, supported by the rising deployment of automated extraction units, liquid handling system, and sample processing platforms across laboratories aiming to streamline workflows and increase throughput consistency. Broader integration of modular workstations into sequencing, diagnostics, and multi-omics workflows reinforces the leading position of this segment.
Software & Services registered the fastest growth at 10.12%, driven by expanding adoption of workflow management tools, cloud-based instrumentation control, and maintenance services that enhance system performance. Growth in digitally coordinated laboratory environments further accelerates uptake of this segment.
Application Insights
Clinical Diagnostics led the application segment with 34.56%, supported by growing utilization of automated preparation systems in molecular testing workflows, including nucleic acid extraction, assay setup, and pathogen detection. Expansion of high throughput diagnostic testing platforms contributes to the continued leadership of this segment.
Genomics & Next-generation Sequencing recorded the fastest growth at 10.45%, propelled by wider incorporation of automation into library preparation, normalization, and sample indexing workflows. Increased sequencing volume across research and commercial laboratories strengthens the rapid expansion of this application area.
End Use Insights
Pharmaceutical & Biotechnology Companies dominated the end use segment with 41.24%, driven by extensive integration of automated platforms into drug discovery, biomarker validation, and large scale molecular screening programs. Consistent upgrades in R&D infrastructure across biopharma facilities sustain this segment’s lead.
Academic & Research Institutes posted the fastest growth at 10.56%, supported by expanded use of automated systems for multi omics projects, training programs, and research-driven assay development. Growth in automation-focused laboratory modernization initiatives enhances adoption across universities and research centers.
End Use Market share (%) in 2025
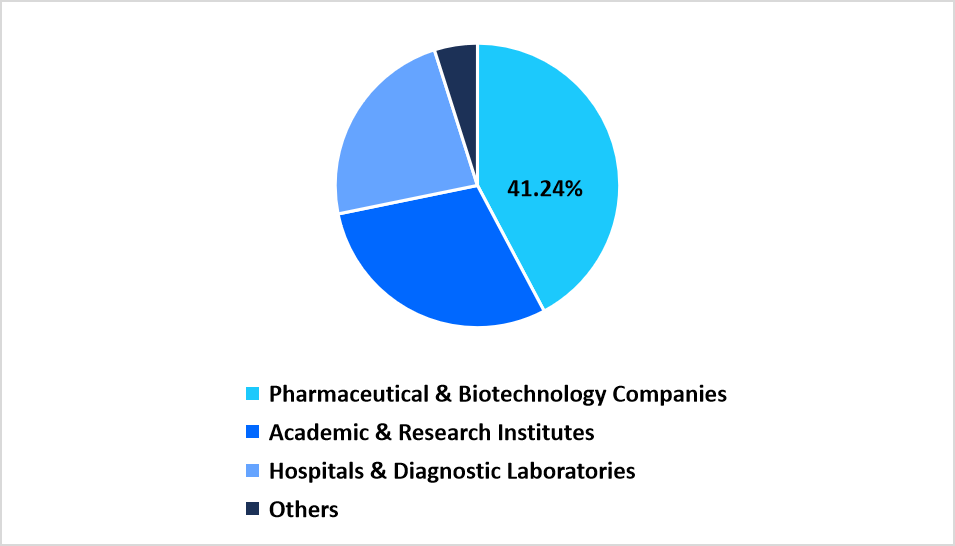
Source: Straits Research
Competitive Landscape
The global automated sample preparation technology market is moderately fragmented, with instrument manufacturers, consumable suppliers, automation platform providers, and service vendors occupying steady positions. These firms expand their presence through portfolio expansion, improvements in sample handling and throughput workflows, strategic partnerships with academic and biopharma laboratories, and broader adoption across genomics, proteomics, clinical diagnostics, and research applications.
PerkinElmer: An emerging market player
PerkinElmer delivers automation platforms, liquid handling systems, and consumables tailored for laboratories aiming to streamline sample processing across genomics, drug discovery, and molecular biology workflows. The company focuses on improving throughput consistency through modular robotic workstations, integrated extraction systems, and workflow-oriented software that simplifies setup and execution. PerkinElmer also strengthens its position by aligning its instruments with assay development, screening applications, and multi-omics workflows, enabling laboratories to process larger sample volumes with reduced manual intervention. Continuous enhancements to system compatibility and consumable design support smoother integration with downstream analytical platforms, reinforcing the company’s role in laboratory automation.
List of Key and Emerging Players in Automated Sample Preparation Technology Market
- AutoGen, Inc.
- Zymo Research Corporation
- Aurora Biomed Inc.
- Hamilton Company
- Agilent Technologies, Inc.
- Hoffmann-La Roche Ltd
- Eppendorf SE
- Promega Corporation
- Bio-Rad Laboratories, Inc.
- MGI Tech Co., Ltd.
- Analytik Jena GmbH+Co. KG
- Thermo Fisher Scientific Inc.
- Tecan Trading AG
- QIAGEN
- PerkinElmer
- Others
Strategic Initiatives
- April 2025: QIAGEN unveiled a strategic initiative to introduce three new sample preparation instruments: QIAsymphony Connect, QIAsprint Connect, and QIAmini, by 2026, designed to advance laboratory automation, boost workflow efficiency, and cater to both high and low throughput laboratory needs.
- May 2025: MGI Tech introduced its next-generation automation portfolio featuring the PrepALL flexible liquid handling system and the upgraded Smart8 platform, designed to streamline laboratory workflows in genomics, drug discovery, and molecular diagnostics, while delivering scalable, AI-enabled automation solutions for both research and clinical labs.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.35 Billion |
| Market Size in 2026 | USD 1.48 Billion |
| Market Size in 2034 | USD 3.10 Billion |
| CAGR | 9.68% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product & Service, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Automated Sample Preparation Technology Market Segments
By Product & Service
- Instruments
- Consumables
- Software & Services
By Application
- Clinical Diagnostics
- Genomics & Next-generation Sequencing
- Proteomics & Metabolomics
- Drug Discovery & Development
- Others
By End Use
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Hospitals & Diagnostic Laboratories
- Other
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































