Blood Clot Retrieval Devices Market Size, Share & Trends Analysis Report By Type (Guidewires, Aspiration devices, Stent retrievers, Others), By Application (Coronary Arteries, Peripheral Arteries, Cerebral Arteries), By End User (Hospitals and Clinics, Ambulatory Surgery Centers, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Blood Clot Retrieval Devices Market Insights
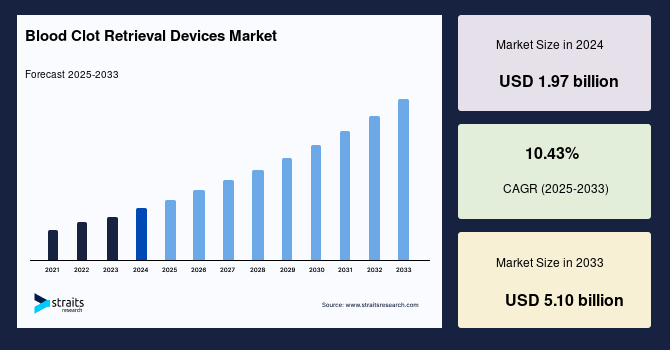
The global blood clot retrieval devices market size was valued at USD 1.97 billion in 2024 and is projected to grow from USD 2.32 billion in 2025 to reach USD 5.10 billion by 2033, exhibiting a CAGR of 10.43% during the forecast period (2025-2033).
Blood clot retrieval devices are advanced medical instruments designed to remove blood clots from blood vessels during procedures aimed at treating conditions like deep vein thrombosis (DVT), pulmonary embolism, or stroke. These devices work by physically extracting or breaking down the clot obstructing the blood vessel, helping to restore normal circulation and preventing further damage to organs and tissues.
The global market is experiencing robust growth, fueled by significant advancements in minimally invasive thrombectomy procedures. Innovations such as stent retrievers, aspiration catheters, and AI-enhanced imaging technologies have significantly improved the efficiency of clot removal, reduced procedural risks, and minimized patient recovery times. These developments are driving the adoption of these devices across various healthcare settings.
The integration of cutting-edge technologies like robotic-assisted thrombectomy, real-time AI analytics, and next-generation aspiration systems is enhancing procedural precision, enabling faster treatment timelines, and increasing success rates. These innovations are particularly transforming stroke care by making clot retrieval procedures safer and more effective, ultimately leading to improved patient outcomes.
Blood Clot Retrieval Devices Market Trends
Shift toward Minimally Invasive Procedures
The rising adoption of minimally invasive techniques in blood clot retrieval is reducing recovery time and complications. Advanced blood clot retrieval devices, such as stent retrievers and aspiration catheters, offer faster and more effective clot removal compared to traditional treatments.
- For instance, in December 2023, Perfuze received FDA clearance for the Millipede 070 Aspiration Catheter and the 2nd generation Millipede 088 Access Catheter, reinforcing the trend toward more efficient, minimally invasive stroke treatments.
This growing shift toward minimally invasive solutions, driven by technological advancements and regulatory approvals, is set to enhance patient outcomes and boost market growth.
Integration of Artificial Intelligence (ai)
The integration of AI into blood clot retrieval devices is revolutionizing thrombectomy procedures by enhancing precision, optimizing real-time decision-making, and improving overall patient outcomes. AI-powered imaging and analytics provide clinicians with faster clot detection, automated navigation assistance, and optimized procedural workflows, reducing treatment delays and increasing success rates.
- For instance, in December 2023, Medanta introduced the Penumbra Lightning 12 F Catheter, an AI-driven device for selective clot removal in endovascular procedures. The AI-powered device minimizes blood loss, reduces complications such as anemia, and expedites patient recovery.
Such developments are expected to fuel market growth by making thrombectomy procedures safer, more accessible, and highly effective in treating life-threatening clot-related conditions.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 1.97 Billion |
| Estimated 2025 Value | USD 2.32 Billion |
| Projected 2033 Value | USD 5.10 Billion |
| CAGR (2025-2033) | 10.43% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
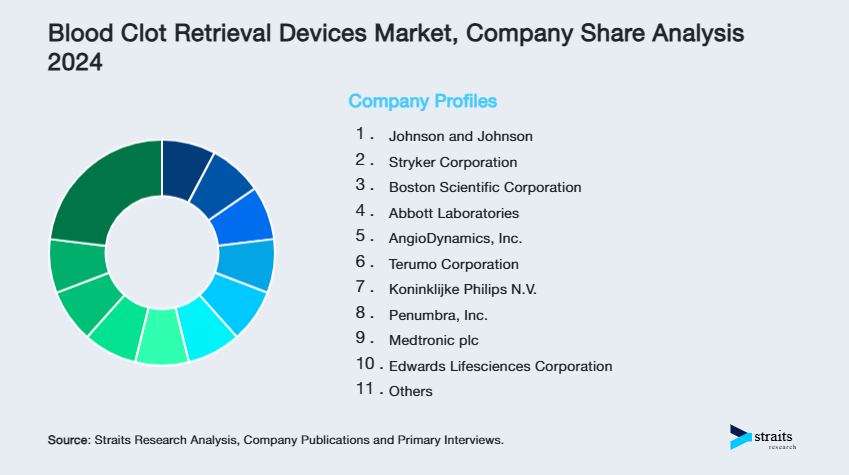
| Key Market Players | Johnson and Johnson, Stryker Corporation, Boston Scientific Corporation, Abbott Laboratories, AngioDynamics, Inc. |
to learn more about this report Download Free Sample Report
Blood Clot Retrieval Devices Market Driving Factors
Rising Cases of Stroke Worldwide
The increasing prevalence of strokes, particularly ischemic strokes, is a key driver of the market. Factors such as sedentary lifestyles, an aging population, and the rising incidence of hypertension and diabetes are contributing to a higher global stroke burden.
For instance,
- In October 2024, the Centers for Disease Control and Prevention (CDC) reported that approximately 87% of all strokes are ischemic, where blood flow to the brain is obstructed, underscoring the need for effective clot retrieval solutions.
- Likewise, the CDC highlighted that every 40 seconds, someone in the U.S. experiences a stroke, with over 795,000 cases annually. Of these, 610,000 are first-time strokes, while 185,000 occur in individuals with a prior history, emphasizing the urgency for rapid and efficient stroke interventions.
As stroke cases continue to rise globally, the demand for blood clot retrieval devices is expected to grow, driving market expansion and encouraging further innovation in minimally invasive thrombectomy procedures.
Growing Technological Advancements
Ongoing advancements in blood clot retrieval technologies are significantly enhancing the efficacy, safety, and accessibility of stroke treatments. Innovations in stent retrievers, aspiration catheters, AI-driven imaging, and robotic-assisted thrombectomy are improving procedural precision, reducing treatment times, and minimizing complications.
- For instance, in April 2024, Expanse ICE announced that the ICE Aspiration System received clearance from the U.S. Food and Drug Administration (FDA). This system represents the latest innovation in aspiration-based thrombectomy, designed to improve clot removal efficiency while minimizing procedural risks.
As next-generation thrombectomy devices continue to evolve, their adoption is expected to enhance treatment success rates, accelerate patient recovery, and drive market growth by making stroke interventions more effective and widely accessible.
Market Restraining Factors
Stringent Regulatory Requirements
The global blood clot retrieval devices market faces significant hurdles due to stringent regulatory frameworks enforced by agencies such as the U.S. FDA, the European Medicines Agency (EMA), and other global health authorities. While these regulations are essential for ensuring patient safety and device efficacy, they also pose challenges for manufacturers.
Companies must navigate lengthy approval processes, extensive clinical trials, and rigorous quality assessments, often leading to delays in product launches and increased compliance costs. Moreover, evolving regulatory standards and the need for post-market surveillance further complicate market entry.
These factors can hinder innovation, limit the availability of next-generation thrombectomy devices, and create barriers for smaller manufacturers, ultimately impacting the pace of market expansion and product accessibility.
Market Opportunity
Adoption of Robotic-Assisted Thrombectomy
The integration of robotic-assisted technology in blood clot retrieval is revolutionizing stroke treatment by enhancing precision, reducing procedure time, and improving patient outcomes. Robotics enables remote-controlled thrombectomy, minimizing human error while expanding access to specialized stroke care in regions with limited neurovascular expertise. This technology also improves catheter navigation and clot removal efficiency, offering greater control during procedures.
- For instance, in May 2024, Rapid Medical achieved the first successful robotic thrombectomy using the Robotic TIGERTRIEVER. The device, which autonomously adapts to a patient's anatomy, successfully treated two patients, marking a major advancement in endovascular stroke treatment.
As robotic-assisted thrombectomy gains traction, it is expected to broaden access to stroke care, improve procedural accuracy, and drive innovation and investment in the global market.
Regional Insights
North America: Dominant Region with 41.5% Market Share
North America holds the largest share in the global blood clot retrieval devices market, driven by the high prevalence of ischemic strokes, rapid adoption of innovative thrombectomy technologies, and advanced healthcare infrastructure. The region benefits from favorable reimbursement policies, strong government support for stroke treatment, and a well-established network of stroke centers.
Moreover, leading market players such as Medtronic, Stryker, and Penumbra continue to invest in research and development, introducing next-generation thrombectomy devices. The rising geriatric population, increasing awareness of stroke management, and growing adoption of AI-assisted clot retrieval technologies further strengthen the market’s growth.
Asia Pacific: Fastest Growing Region with the Highest Market Cagr
Asia-Pacific is projected to register the fastest CAGR, driven by the rising incidence of ischemic strokes, increasing healthcare expenditure, and growing adoption of minimally invasive thrombectomy procedures. Supportive government initiatives, expanding healthcare infrastructure, and rising investments in neurovascular care are fueling market expansion. The region is also experiencing greater accessibility to advanced stroke treatment technologies, collaborations with global medical device manufacturers, and an increasing number of specialized stroke centers, further accelerating market growth.
Countries Insights
- U.S. - The U.S. dominates the blood clot retrieval devices industry due to high investments in R&D, rapid technological advancements, and frequent product launches. The presence of major players like Medtronic, Stryker, and Penumbra drives innovation. In June 2024, MicroVention, Inc., a subsidiary of Terumo Corporation, launched the ERIC retrieval device, enhancing thrombus control, reducing procedure time, and offering versatile thrombectomy options. Strong reimbursement policies and a well-established stroke care infrastructure further boost market growth.
- Germany– Germany’s market for blood clot retrieval devices is one of the largest in Europe, driven by the launch of innovative products in the country. For instance, in September 2024, CERENOVUS, Inc., a part of Johnson & Johnson MedTech, launched EMBOGUARD, a next-generation balloon guide catheter in Germany for endovascular procedures, including acute ischemic stroke treatment. This innovation enhances clot retrieval efficiency, improves procedural outcomes, and reinforces the adoption of advanced thrombectomy technologies.
- UK- The UK market is expanding due to the increasing incidence of ischemic strokes, rising adoption of minimally invasive thrombectomy, and strong healthcare infrastructure. NHS guidelines actively support mechanical thrombectomy, making it a standard stroke care procedure. Government funding for stroke treatment advancements, along with the expansion of specialized stroke centers, is further boosting the demand for advanced blood clot retrieval technologies.
- Canada- Canada's market for blood clot retrieval devices is growing due to the rising prevalence of ischemic strokes, increasing mechanical thrombectomy adoption, and strong government initiatives. The expansion of specialized stroke centers and favorable reimbursement policies enhance access to advanced treatments. Additionally, advancements in AI-driven imaging and robotic-assisted thrombectomy are further accelerating market growth, ensuring more precise and efficient clot retrieval procedures to improve patient outcomes.
- India – India’s blood clot retrieval devices market is expanding due to a rising stroke burden, increasing awareness, and supportive government initiatives. In March 2022, Medtronic plc launched India’s first dedicated registry to collect real-world data on revascularization device use in acute ischemic stroke patients. This initiative is helping enhance clinical insights, improve treatment strategies, and boost the adoption of advanced thrombectomy technologies, positioning India as a fast-growing market.
- China– China’s market growth is fueled by a rising incidence of ischemic strokes, increasing healthcare investments, and expanding neurovascular treatment centers. Government-led stroke care initiatives and collaborations with domestic and global medical device manufacturers are strengthening the market. The growing adoption of minimally invasive thrombectomy procedures and AI-assisted stroke management further drives demand, positioning China as a key player in the Asia-Pacific region’s thrombectomy market expansion.
- Japan - Japan is witnessing substantial growth in the market due to high R&D investments, technological advancements, and favorable regulatory policies. In 2024, Inari Medical, Inc. received national reimbursement approval from Japan’s Ministry of Health, Labor, and Welfare (MHLW) for its ClotTriever Thrombectomy System for DVT, underscoring Japan’s commitment to enhancing stroke care and expanding access to next-generation thrombectomy devices.
Segmentation Analysis
The global blood clot retrieval devices market is segmented into type, application, and end-user.
By Type
Stent retrievers dominate the global market due to their high success rates in mechanical thrombectomy, faster recanalization, and superior patient outcomes. These devices mechanically engage and remove clots, restoring blood flow in ischemic stroke patients. Their widespread adoption is driven by technological advancements, growing stroke incidence, and increasing preference for minimally invasive procedures. Moreover, favorable clinical guidelines and expanding stroke care infrastructure continue to support the segment’s growth.
By Application
The cerebral arteries segment holds the largest share in the global market, primarily due to the high prevalence of ischemic strokes, which account for the majority of global stroke cases. When a clot obstructs a major brain artery, rapid intervention is crucial to prevent severe disability or death. The growing adoption of mechanical thrombectomy as a frontline treatment, increased awareness of stroke management, and expanding healthcare infrastructure are further fueling demand for cerebral artery interventions.
By End User
Hospitals remain the largest end-user segment in the global market, owing to their specialized stroke care units, access to skilled healthcare professionals, and high patient inflow for thrombectomy procedures. Equipped with advanced imaging technologies, neurovascular intervention units, and emergency stroke response teams, hospitals play a crucial role in treating stroke patients. The increasing adoption of mechanical thrombectomy in hospital settings, alongside supportive reimbursement policies, government funding, and infrastructure expansion, continues to drive market growth.
Company Market Share
Key players in the global blood clot retrieval devices market are actively adopting strategic business approaches to enhance their market position. These strategies include mergers and acquisitions, product innovations, regulatory approvals, geographical expansions, and partnerships with healthcare institutions. Companies are also investing heavily in R&D to develop next-generation thrombectomy devices with improved safety and efficiency.
Penumbra, Inc.: An Emerging Player in the Global Market
Penumbra, Inc. is a leading provider of blood clot retrieval devices, specializing in aspiration-based thrombectomy technologies for treating ischemic stroke, deep vein thrombosis (DVT), and arterial thrombosis. The company’s Lightning Intelligent Aspiration and Indigo systems utilize AI-driven clot detection and real-time feedback to enhance procedural precision and improve patient outcomes.
Recent developments at Penumbra, Inc. include:
- In June 2023, Penumbra, Inc. received FDA clearance and launched Lightning Bolt™ 7, an advanced arterial thrombectomy system. It features modulated aspiration technology, combining Lightning Intelligent Aspiration with a microprocessor algorithm to rapidly remove large, fibrous blood clots with minimal blood loss, addressing conditions like acute limb ischemia and visceral occlusions.
List of Key and Emerging Players in Blood Clot Retrieval Devices Market
- Johnson and Johnson
- Stryker Corporation
- Boston Scientific Corporation
- Abbott Laboratories
- AngioDynamics, Inc.
- Terumo Corporation
- Koninklijke Philips N.V.
- Penumbra, Inc.
- Medtronic plc
- Edwards Lifesciences Corporation
- BIOTRONIK
- Argon Medical Devices
- Straub Medical
- B. Braun SE
- Perfuze Ltd.
to learn more about this report Download Market Share
Recent Developments
- September 2024 – Argon Medical Devices launched the CLEANER Vac Thrombectomy System for peripheral venous clot removal. This innovation enhances efficacy in thrombus clearance, supporting the growing adoption of minimally invasive thrombectomy solutions.
Analyst Opinion
As per our analyst, the global market is set for robust growth, primarily fueled by the increasing incidence of ischemic strokes and the expanding use of minimally invasive thrombectomy procedures. Technological innovations, such as AI-driven imaging, robotic-assisted thrombectomy, and next-generation aspiration systems, are playing a pivotal role in enhancing procedural precision, reducing complications, and improving patient outcomes.
Despite the challenges posed by stringent regulatory requirements and high product development costs, advancements in intelligent aspiration technologies are driving greater efficiency in clot removal. Moreover, emerging markets in Asia-Pacific present significant opportunities for expansion, supported by rising stroke prevalence, improving healthcare infrastructure, and growing access to advanced neurovascular interventions.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 1.97 Billion |
| Market Size in 2025 | USD 2.32 Billion |
| Market Size in 2033 | USD 5.10 Billion |
| CAGR | 10.43% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Blood Clot Retrieval Devices Market Segments
By Type
- Guidewires
- Aspiration devices
- Stent retrievers
- Others
By Application
- Coronary Arteries
- Peripheral Arteries
- Cerebral Arteries
By End User
- Hospitals and Clinics
- Ambulatory Surgery Centers
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Jay Mehta
Research Analyst
Jay Mehta is a Research Analyst with over 4 years of experience in the Medical Devices industry. His expertise spans market sizing, technology assessment, and competitive analysis. Jay’s research supports manufacturers, investors, and healthcare providers in understanding device innovations, regulatory landscapes, and emerging market opportunities worldwide.










































