Cell Therapy Raw Materials Market Size, Share & Trends Analysis Report By Product (Media, Sera, Cell Culture Supplements, Antibodies, Reagents & Buffers, Others), End Use (Biopharmaceutical & Pharmaceutical Companies, CROs & CMOs, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Cell Therapy Raw Materials Market Overview
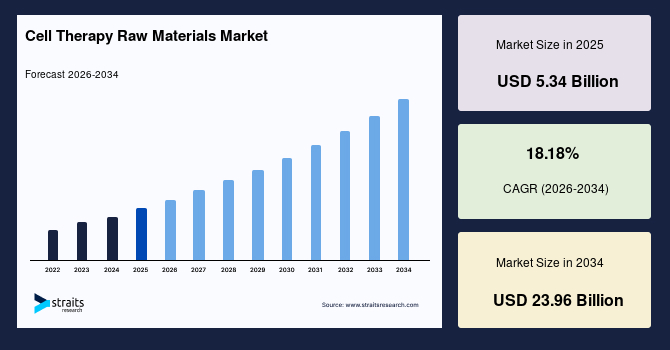
The global cell therapy raw materials market size is estimated at USD 5.34 billion in 2025 and is projected to reach USD 23.96 billion by 2034, growing at a CAGR of 18.18% during the forecast period. Sustained growth of the market is propelled by the rising transition of early-stage cell therapy pipelines into multicentre trial formats, which increases demand for standardized raw materials that maintain consistent performance across dispersed manufacturing sites and diverse production environments.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 90.35%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 20.18%.
- By Product, the Cell Culture Supplements segment dominated the market with a revenue share of 27.78%.
- By End Use, the Biopharmaceutical & Pharmaceutical Companies segment dominated the market with a revenue share of 55.64%.
- The U.S. dominates the global cell therapy raw materials market, valued at USD 2.00 billion in 2024 and reaching USD 2.36 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
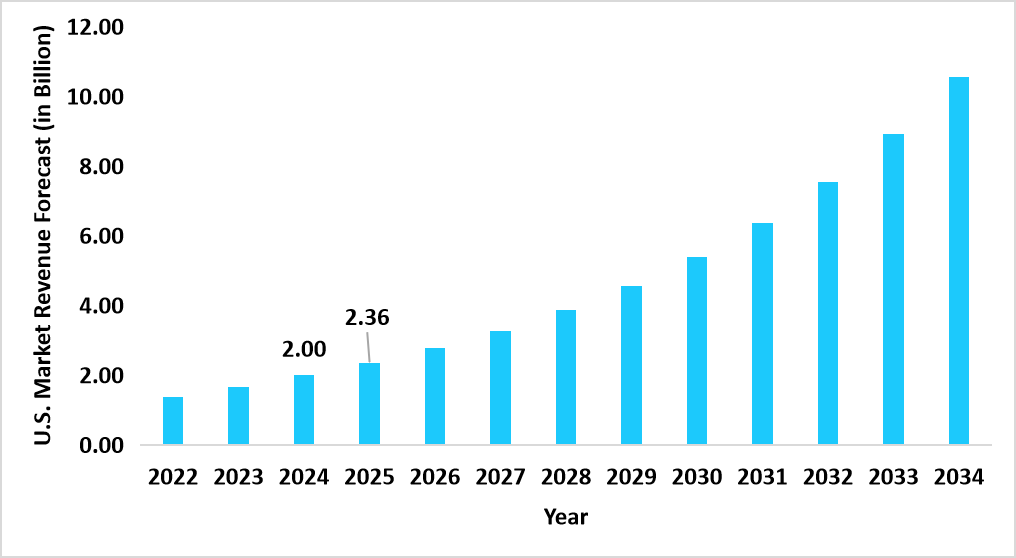
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 5.34 billion
- 2034 Projected Market Size: USD 23.96 billion
- CAGR (2025 to 2034): 18.18%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The cell therapy raw materials market comprises all essential inputs used to culture, expand, modify, and preserve therapeutic cell populations across research, clinical development, and commercial manufacturing. These materials include media, sera, cell culture supplements, antibodies, reagents, buffers, and various auxiliary components that support controlled growth and functional stability of immune cells, stem cells, and engineered cell lines. Demand is primarily driven by biopharmaceutical and pharmaceutical companies advancing autologous and allogeneic therapy pipelines, alongside rising procurement from CROs, CMOs, and other specialized facilities involved in process development, analytics, and fill–finish operations. As global cell therapy programs scale, the market continues to evolve toward higher-quality, GMP-aligned raw materials that enable consistent production across diverse therapeutic platforms.
Latest Market Trends
Rising Use of Donor-to-Donor Variability Mapping Tools
A notable trend is the growing focus on platforms that map variability across donor-derived cell sources. Developers are adopting analytical suites that track metabolic behavior, surface markers, and growth patterns across different donor batches to refine raw material selection. This approach supports more predictable outcomes for cell expansion runs and strengthens material qualification workflows for autologous programs.
Expansion of In-House Raw Material Characterization Labs Across ATMP Facilities
An emerging trend is the steady build-out of dedicated characterization units within advanced therapy manufacturing sites. These labs perform a deeper assessment of cytokines, supplements, sera alternatives, and processed reagents before they move into production lines. Facilities are prioritizing on-site analytics to manage purity, bioactivity, and consistency checks, creating a stronger quality gate for raw materials entering clinical-grade operations.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 5.34 Billion |
| Estimated 2026 Value | USD 6.29 Billion |
| Projected 2034 Value | USD 23.96 Billion |
| CAGR (2026-2034) | 18.18% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific, Merck KGaA, Sartorius AG, STEMCELL Technologies, Lonza |
to learn more about this report Download Free Sample Report
Cell Therapy Raw Materials Market Driver
Growing Shift Toward Custom Formulation Pipelines for Cell-Specific Requirements
A key driver comes from the rising preference for tailored raw material formulations designed for distinct cell types. Therapy developers are working closely with suppliers to build custom blends of media supplements, cytokines, and activation agents that align with unique culture demands. This trend increases supplier–developer collaboration and boosts demand for specialized raw material configurations across both immune-cell and stem-cell platforms.
Market Restraint
Limited Availability of High-Volume GMP Inputs for Large Allogeneic Programs
A restraint arises from supply constraints linked to high-volume allogeneic pipelines. Large-scale platforms require bulk quantities of cytokines, serum alternatives, and high-grade reagents that are produced under strict GMP conditions. Production lead times and batch-size limits restrict availability, which slows expansion plans for manufacturers preparing commercial-scale runs.
Market Opportunity
Rising Expansion of Cryo-Qualified Raw Material Supply Chains for Global Distribution
An opportunity develops from the widening build-out of cryo-qualified logistics networks that support the movement of temperature-sensitive raw materials across global therapy hubs. Suppliers are expanding controlled transport systems for cytokines, sera alternatives, media components, and frozen intermediates to maintain stability across long routes. As cross-border clinical programs scale, demand rises for raw materials supported by validated cold-chain delivery, creating stronger uptake for suppliers that offer temperature-assured distribution models.
Regional Analysis
North America holds the largest share of 48.78% in the cell therapy raw materials market, supported by the broad adoption of GMP-grade inputs across commercial cell therapy programs, early-stage trials, and advanced biologics facilities. The region’s growth is shaped by the expansion of cell-processing centers, continuous upgrades in manufacturing infrastructure, and rising procurement of T-cell, stem-cell, and viral-vector raw materials across the U.S. and Canada. Developers and CDMOs across the region maintain an extensive footprint for culture media, cytokines, reagents, and closed-system consumables used across autologous and allogeneic therapy lines.
The U.S. expands its position through federal and state programs that upgrade cell-therapy manufacturing sites and strengthen domestic supply ecosystems. These initiatives increase procurement of GMP-grade media, serum-free formulations, cell-isolation materials, and vector-production inputs across major therapy developers, academic manufacturing units, and commercial producers.
Asia Pacific Market Insights
Asia Pacific accounts for a growing CAGR of 20.18% driven by the rising establishment of cell-therapy manufacturing clusters across East Asia and South Asia. Regional hubs continue to scale specialized facilities for stem-cell banking, immune-cell production, and viral-vector synthesis, creating steady demand for GMP raw materials. Countries including China, South Korea, Japan, India, and Southeast Asian nations expand procurement of media, cytokines, and ancillary materials as clinical pipelines widen and regulatory authorities strengthen quality frameworks.
Growth in India is influenced by national life-science programs and pharmaceutical-manufacturing expansion efforts that encourage modernization of biologics infrastructure. These initiatives support increased use of compliant raw materials across cell-culture, expansion, and fill-finish operations in both public and private units.
Pie Chart: Regional Market Share, 2025
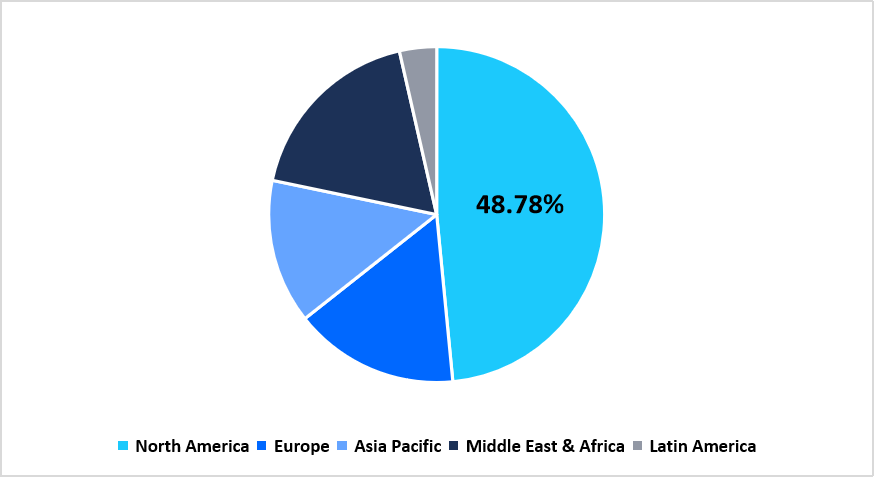
Source: Straits Research
Europe Market Insights
Europe records consistent expansion supported by a region-wide focus on harmonized quality frameworks for advanced therapy manufacturing. Strong regulatory alignment across member states increases adoption of GMP-grade media, specialized reagents, and cell-processing materials across research centers, CDMOs, and commercial producers. Cross-border cooperation within European biotech networks accelerates the development of standardized workflows that rely on high-purity raw materials for T-cell, MSC, and pluripotent stem-cell programs.
Germany Market: Growth in Germany is influenced by continuous federal investment in modern biologics and ATMP production sites. These initiatives strengthen demand for controlled cell-culture inputs, chemically defined media, and ancillary reagents used across monoclonal antibody, vaccine, and cell-therapy operations.
Middle East and Africa Market Insights
Middle East and Africa observe a rising uptake of cell-therapy raw materials supported by national biotechnology programs and growing partnerships with global therapy developers. Emerging biomanufacturing hubs in the region procure higher volumes of media, serum alternatives, viral-vector raw materials, and cold-chain consumables to support new production lines for ATMPs and advanced biologics. Government-industry collaborations contribute to the rapid development of cell-therapy capabilities in selected urban centers.
Saudi Arabia shows progress driven by national strategies focused on expanding domestic biotechnology capacity. These programs lead to higher installation of compliant manufacturing units, boosting demand for raw materials used in cell expansion, cell-separation workflows, and regulatory-aligned production cycles.
Latin America Market Insights
Latin America reports increasing utilization of cell-therapy raw materials as regional institutions upgrade capacity for vaccines, regenerative therapies, and clinical-grade biologics. Collaborative projects across leading countries support wider adoption of standardized materials, including serum-free media, cytokines, and viral-vector components. Research institutes and private manufacturers across the continent integrate improved material procurement channels to support their growing cell-therapy pipelines.
In Brazil, growth is influenced by coordinated national and state programs aimed at reinforcing biologics and ATMP production infrastructure. These efforts contribute to the broader adoption of GMP-grade raw materials across federal institutes and regional therapeutic manufacturing clusters.
Product Insights
Cell Culture Supplements dominate the market in 2025 with a share of 27.78%. This position reflects broad utilization of supplements that support cell expansion, activation, and maintenance across clinical and commercial manufacturing lines. As developers advance autologous and allogeneic platforms, demand rises for supplements that maintain consistent growth conditions across diverse cell types. Their wide application across research, process development, and regulated production supports the leading position of this segment.
Media is the fastest-growing product segment with a growth share of 19.21%. Growth in this category is driven by wider adoption of specialized media tailored for immune cells, stem cells, and vector-producing lines. As therapy pipelines advance toward higher-volume production, facilities expand procurement of serum-free and chemically defined formulations, leading to steady annual growth for this segment.
End Use Insights
Biopharmaceutical and Pharmaceutical Companies dominate the market in 2025 with a share of 55.64%. Large manufacturers lead raw material consumption due to expanding pipelines for cell-based therapies and continuous upgrades in their production lines. These companies increase sourcing of media, supplements, sera, and reagents to support GMP-grade workflows, ranging from early development to commercial-scale manufacturing, which sustains the dominant position of this segment.
CROs and CMOs represent the fastest-growing end-use segment with 19.54%. Expansion of outsourced development and manufacturing encourages higher procurement of raw materials across contract facilities. Growing project volumes from therapy developers drive the requirement for standardized materials used in cell expansion, vector production, and quality-controlled processing, contributing to strong segment growth through the forecast period.
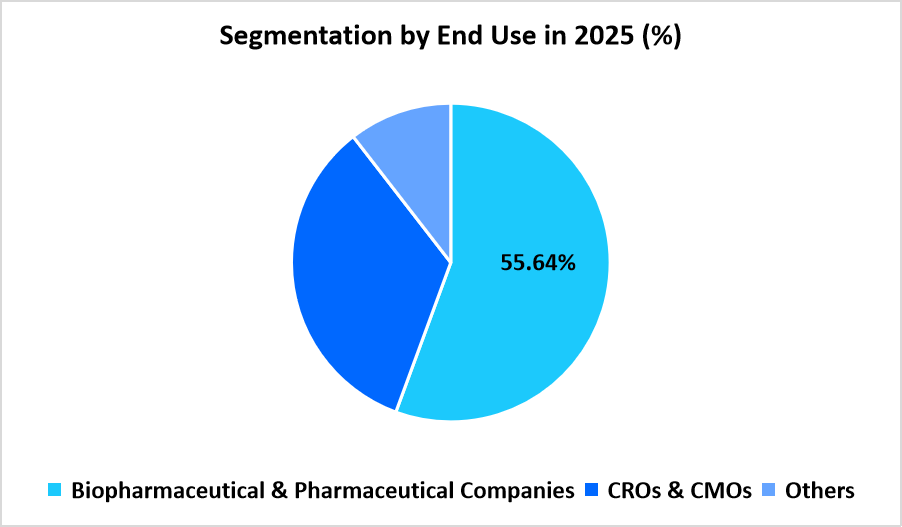
Source: Straits Research
Competitive Landscape
The global cell therapy raw materials market is moderately fragmented, with competition distributed across major life-science manufacturers, specialized cell-processing reagent providers, GMP-grade material suppliers, and emerging biotech companies focused on next-generation cell-culture components. Market dynamics are shaped by continuous advancements in regenerative medicine, growing clinical trial volumes, and increasing demand for high-purity, regulatory-compliant raw materials used in cell expansion, activation, isolation, and preservation.
Merck KGaA : An emerging market player
Merck KGaA focused on expanding its raw-material ecosystem through chemically defined media, viral vector raw materials, and advanced purification reagents. With its integrated quality and regulatory platform (Emprove), Merck supported accelerated development timelines for cell and gene therapy manufacturers.
List of Key and Emerging Players in Cell Therapy Raw Materials Market
- Thermo Fisher Scientific
- Merck KGaA
- Sartorius AG
- STEMCELL Technologies
- Lonza
- Miltenyi Biotec
- Bio-Techne
- BD
- GE HealthCare
- Corning Incorporated
- Sartorius CellGenix GmbH
- Charles River Laboratories
- ACROBiosystems
- Actylis
- RoosterBio, Inc.
- PromoCell
- Repligen Corporation
- Eppendorf SE
- PBS Biotech, Inc.
- Others
Strategic Initiatives
- December 2024: BioCentriq signed a long-term lease for a new manufacturing facility in Princeton, NJ, which served as its headquarters. The USD 12 million investment enhanced its capabilities in cell therapy development and production.
- October 2024: Thermo Fisher Scientific established a bioprocess design center in Genome Valley, Hyderabad. This move reflected the growing demand in the market.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 5.34 Billion |
| Market Size in 2026 | USD 6.29 Billion |
| Market Size in 2034 | USD 23.96 Billion |
| CAGR | 18.18% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Cell Therapy Raw Materials Market Segments
By Product
- Media
- Sera
- Cell Culture Supplements
- Antibodies
- Reagents & Buffers
- Others
End Use
- Biopharmaceutical & Pharmaceutical Companies
- CROs & CMOs
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































