Dengue Vaccine Market Size, Share & Trends Analysis Report By Product (Dengvaxia, Qdenga, Others), By Route of Administration (Oral, Parenteral, Others), By End User (Government & Public Health Agencies, Hospitals & Specialty Clinics, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Dengue Vaccine Market Overview
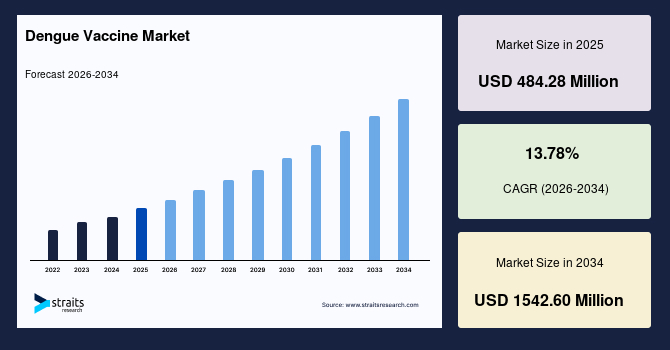
The global dengue vaccine market size is estimated at USD 484.28 million in 2025 and is projected to reach USD 1542.60 million in 2034, growing at a CAGR of 13.78% during the forecast period. The remarkable growth of the market is due to the rising expansion of national dengue control programs that increasingly incorporate vaccination as a core prevention strategy, supported by broader surveillance networks that provide clearer insight into seasonal outbreaks and population-level exposure patterns.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 44.24% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 15.78%.
- Based on Product, the Dengvaxia segment dominates the market with a revenue share of 85.67%.
- Based on the Route of Administration, the parenteral segment dominates the market with a revenue share of 66.52%.
- Based on End User, the hospitals and specialty clinics segment dominates the market with a revenue share of 75.12%.
- The U.S. dominates the global market, valued at USD 170.78 million in 2024 and reaching USD 193.57 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
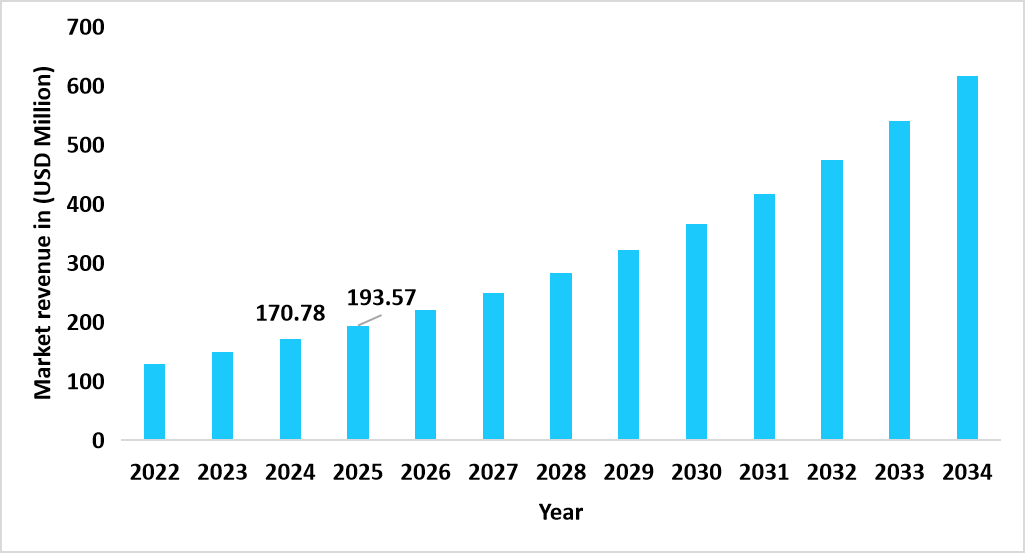
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 484.28 million
- 2034 Projected Market Size: USD 1542.60 million
- CAGR (2025 to 2034): 13.78%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The dengue vaccine market encompasses the development, production, and distribution of vaccines designed to prevent dengue virus infection across diverse population groups. The market spans different routes of administration, including oral, parenteral, and emerging alternative delivery methods aimed at expanding accessibility. Commercially available products such as Dengvaxia and Qdenga form the core of current deployment, while additional candidates progress through research pipelines. End users include government and public health agencies that execute mass immunization programs, hospitals and specialty clinics providing individual vaccination services, and other institutional settings involved in disease prevention. Collectively, the market functions within a global framework shaped by rising dengue incidence, evolving immunization strategies, and ongoing efforts to strengthen preparedness in endemic and travel-associated regions.
Latest Market Trends
Shift from Uniform Vaccination Schedules to Serostatus Guided Immunization Strategies
The dengue vaccine market continues its transition from long-standing age-based vaccination schedules toward approaches that classify individuals by serostatus prior to receiving a dose. Earlier programs relied on broad eligibility criteria, but current strategies emphasize screening tools that detect past exposure and categorize recipients with greater clarity. This movement encourages vaccination pathways that align with the immune background across diverse communities. As more countries explore structured screening frameworks, discussions on dose timing, population targeting, and campaign rollout grow more refined, creating a gradual shift toward precision-focused immunization planning.
Shift from Vector Control Centered Programs to Integrated Vaccine Plus Surveillance Frameworks
Public health systems are moving away from strategies dominated by mosquito control toward integrated programs that combine vaccination, real-time case monitoring, and seasonal risk forecasting. Traditional efforts prioritized environmental interventions, whereas current models place greater weight on coordinated actions across epidemiology teams, meteorological units, local clinics, and national vaccine planners. Enhanced mapping of transmission clusters, combined with the strategic release of vaccination campaigns during rising case periods, strengthens preparedness. This shift supports long term planning for regions that encounter recurring dengue cycles and deepens alignment between prevention and response systems.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 484.28 Million |
| Estimated 2026 Value | USD 549.27 Million |
| Projected 2034 Value | USD 1542.60 Million |
| CAGR (2026-2034) | 13.78% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Sanofi, Takeda Pharmaceutical Company Limited, Merck & Co., Inc. (pipeline), Johnson & Johnson (pipeline), Panacea Biotec (pipeline) |
to learn more about this report Download Free Sample Report
Dengue Vaccine Market Driver
Expansion of Cross-Border Travel Generating Rising Demand for Pre-Departure Vaccination
The growing movement of travelers across regions with active dengue transmission continues to drive interest in pre-departure vaccination services. Travel medicine centers report increasing consultations related to dengue planning, particularly for individuals visiting tropical climates during peak seasons. As migration for business, education, and tourism expands, the number of people seeking preventive immunization before travel continues to rise. This dynamic stimulates steady demand for existing vaccines and strengthens the market foundation for upcoming candidates expected to enter global travel clinics.
Market Restraint
Variation in Diagnostic Confirmation Rates Creating Delays in Vaccination Decisions
Diagnostic capacity for dengue remains uneven across different healthcare systems, which affects the speed at which suspected cases receive confirmation. Regions with slower laboratory processing or limited access to molecular tests experience delays in verifying infections, complicating the timing of vaccination decisions for both individuals and communities. These inconsistencies constrain public health planning during periods of rising case numbers and hinder the rapid deployment of vaccination efforts, especially in areas with cyclical outbreaks where timely action is central to reducing disease spread.
Market Opportunity
Expansion of Public-Private Partnerships Supporting Large-Scale Vaccine Distribution Models
Collaborations among government authorities, non-governmental organizations, and vaccine developers continue to grow, creating structured pathways for the wide distribution of dengue vaccines. These alliances strengthen procurement mechanisms, streamline logistics, and reinforce cold chain systems across urban and remote zones. As public health agencies aim to prepare for broader immunization efforts, coordinated partnerships allow smoother integration of vaccines into national programs. This environment opens new avenues for large scale deployment, particularly in regions with rising dengue awareness and interest in population-level prevention strategies.
Regional Analysis
North America maintains a dominating position in the dengue vaccine landscape with a 44.24% share due to ongoing public health preparedness, expanded travel immunization programs, and continued investments in vaccine research across academic and private institutions. Adoption of structured surveillance systems supports early detection of imported dengue cases and sustains regional interest in preventive immunization strategies. Partnerships between government agencies and vaccine developers contribute to steady progress in vaccination planning across urban centers.
The U.S. dengue vaccine market develops through periodic updates in vaccination guidance for travellers, continued research funding for tetravalent vaccine candidates, and coordination among federal agencies monitoring vector-borne infections. Expansion of clinical trial sites across universities strengthens long-term development of new candidates, while state-level programs reinforce awareness among residents traveling to endemic regions.
Asia Pacific Market Insights
Asia Pacific reflects rapid expansion with a 15.78% CAGR driven by the high burden of dengue infections, wider acceptance of preventive vaccination strategies, and continuous increases in national immunization investments across India, Indonesia, Thailand, Vietnam, and the Philippines. Regional ministries advance procurement frameworks for upcoming vaccine candidates and emphasize coordinated vector control measures that complement immunization campaigns. Urban growth and climatic variation across tropical zones sustain a strong demand for preventive solutions.
The China dengue vaccine market progresses through rising provincial-level surveillance activities, the introduction of urban vaccination pilot programs, and local manufacturing partnerships that support future vaccine supply. Expansion of research institutes engaged in vector-borne disease studies strengthens long-term interest in domestic vaccine development, while regional centers prepare for larger scale evaluations of new candidates.
Pie Chart: Regional Market Share, 2025
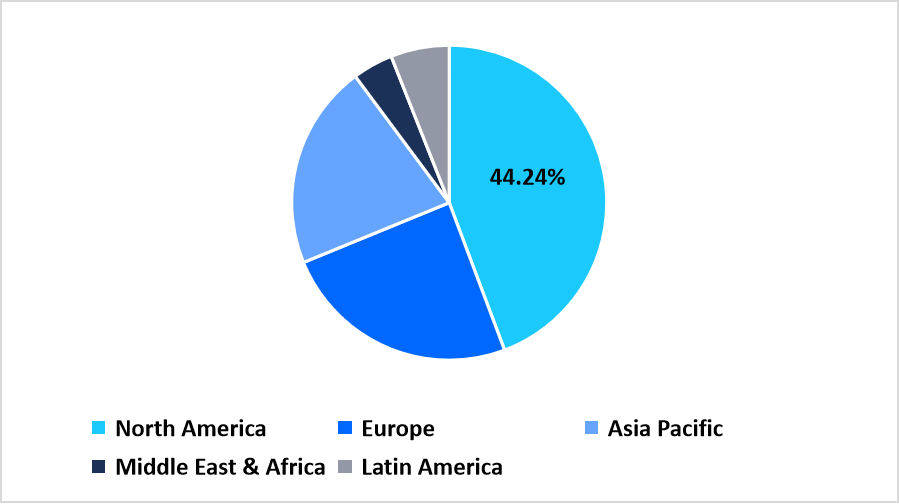
Source: Straits Research
Europe Market Insights
Europe advances its market through unified travel medicine policies, coordinated disease monitoring systems, and expansion of vaccination services for outbound travellers visiting endemic regions. Collaborative work among national public health institutes strengthens guidance on vector-borne risks, while regional clinical networks contribute to assessments of vaccine performance across diverse populations. Growth in international mobility continues to raise interest in preventive vaccination for high-exposure groups.
The UK dengue vaccine market grows through expanded travel health clinics, continued evaluation of vaccination requirements for long-distance travelers, and partnerships between universities and global research groups studying dengue epidemiology. Policy updates encourage structured consultation pathways for individuals planning visits to high transmission areas, strengthening demand for available and upcoming vaccine options.
Middle East and Africa Market Insights
The Middle East and Africa region develops its market through urbanization-driven mosquito expansion, increased awareness of arboviral infections, and gradual enhancement of immunization departments across Gulf and African nations. Cross-border collaborations support surveillance programs while investments in laboratory capacity improve confirmation of dengue circulation in emerging hotspots. Regional travel to endemic countries fosters additional interest in preventive vaccination.
The South Africa dengue vaccine market evolves through national vector monitoring programs, public health training focused on arboviral diseases, and partnerships with international agencies studying mosquito-borne transmission patterns. Expansion of diagnostic laboratories across major provinces contributes to clearer case detection, sustaining interest in future vaccine uptake among at-risk populations.
Latin America Market Insights
Latin America holds a central role in the global market due to high infection rates, long-standing public health engagement, and continuous governmental investment in immunization research. Regional programs encourage phased introduction of new vaccine candidates, while cross-ministry collaborations aim to coordinate surveillance across Brazil, Colombia, Peru, and Mexico. Expansion of municipal healthcare infrastructure strengthens capacity for organized vaccination activities.
The Argentine dengue vaccine market advances through periodic revisions in national dengue response plans, broader inclusion of dengue vaccination discussions within community health programs, and academic studies assessing dengue epidemiology across northern provinces. Expansion of regional bioscience capabilities supports domestic participation in clinical research and increases readiness for future vaccine distribution.
Product Insights
The Dengvaxia segment dominates with 85.67%, supported by its established regulatory presence in selected countries and continued inclusion in controlled vaccination programs for documented seropositive populations. Ongoing post-marketing assessments reinforce utilization where guidelines remain active.
The Qdenga segment records the fastest growth at 14.45%, driven by expanding registrations across multiple regions and rising procurement interest among national agencies preparing for program-level deployment. Wider evaluation across diverse age groups contributes to the growth of this product line.
Route of Administration Insights
The parenteral segment dominates with 66.52%, reflecting extensive use of injectable vaccines across public health campaigns, travel clinics, and hospital-based immunization units. This route maintains priority due to structured administration procedures applied across controlled settings.
The oral segment records the fastest growth at 14.32%, driven by rising interest in vaccine delivery approaches that aim to widen accessibility and simplify administration in large population programs. Continued evaluation of oral formulations across research settings supports the advancement of this category.
End User Insights
The hospitals and specialty clinics segment dominates with 75.12%, benefitting from structured vaccine administration pathways managed through clinical units, travel medicine centers, and outpatient departments. Increased patient flow across institutional settings strengthens the prominence of this segment.
The government and public health agencies segment records the fastest growth at 14.68%, influenced by rising national-level planning for dengue prevention, expansion of procurement frameworks, and greater emphasis on coordinated vaccination strategies during high transmission periods.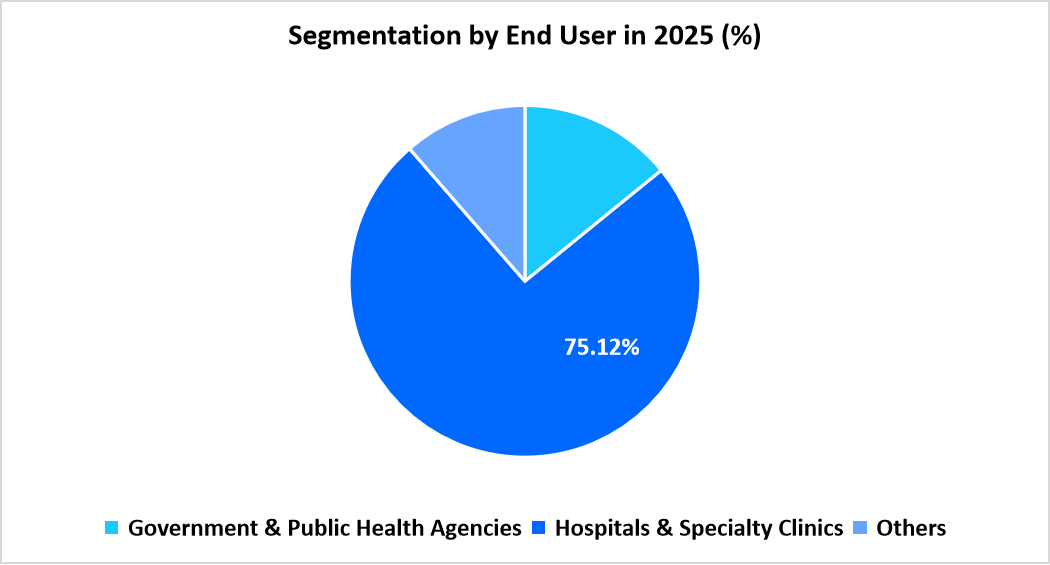
Source: Straits Research
Competitive Landscape
The dengue vaccine market is characterized by a mix of long-standing vaccine manufacturers, regional biologics producers, and research-driven biotechnology organizations that continue to expand development programs across multiple vaccine platforms, including live attenuated, inactivated, subunit, and nucleic acid-based candidates.
Inovio Pharmaceuticals: An emerging market player
Inovio Pharmaceuticals pursued the development of a DNA-based dengue vaccine candidate supported by its nucleic acid delivery platform. In 2024 and 2025, the company engaged in early-stage clinical studies and continued refinement of dosing strategies for populations in dengue endemic regions.
List of Key and Emerging Players in Dengue Vaccine Market
- Sanofi
- Takeda Pharmaceutical Company Limited
- Merck & Co., Inc. (pipeline)
- Johnson & Johnson (pipeline)
- Panacea Biotec (pipeline)
- Biological E. Limited (pipeline)
- Serum Institute of India Pvt. Ltd. (pipeline)
- VBI Vaccines Inc. (pipeline)
- Medigen Vaccine Biologics(pipeline)
- Others
Strategic Initiatives
- June 2025: Merck (known as MSD outside the U.S. and Canada) announced that it had begun the MOBILIZE-1 Phase 3 clinical trial to assess the safety, immunogenicity, and efficacy of a single dose of the investigational quadrivalent vaccine V181 for prevention of dengue.
- May 2025: In partnership with Biological E. Limited, the Japanese pharmaceutical company Takeda Pharmaceutical Company said it intended to introduce its dengue vaccine QDENGA in India the following year. The company hoped to obtain a license by 2026 and reported that local clinical trials and regulatory processes were underway.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 484.28 Million |
| Market Size in 2026 | USD 549.27 Million |
| Market Size in 2034 | USD 1542.60 Million |
| CAGR | 13.78% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Route of Administration, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Dengue Vaccine Market Segments
By Product
- Dengvaxia
- Qdenga
- Others
By Route of Administration
- Oral
- Parenteral
- Others
By End User
- Government & Public Health Agencies
- Hospitals & Specialty Clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.























