Enzyme Replacement Therapy Market Size, Share & Trends Analysis Report By Product (Agalsidase Beta, Imiglucerase, Velaglucerase Alfa, Idursulfase, Galsulfase, Laronidase, Others), By Disease (Gaucher Disease, Fabry Disease, Pompe Disease, Mucopolysaccharidosis, Exocrine Pancreatic Insufficiency (EPI), Others), By End-User (Hospitals, Infusion Centers and Home Healthcare Setting), By Route of Administration (Oral, Parenteral) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Enzyme Replacement Therapy Market Size
The global enzyme replacement therapy market size was valued at USD 10.12 billion in 2024 and is projected to grow from USD 10.95 billion in 2025 to reach USD 20.62 billion by 2033, exhibiting a CAGR of 8.23% during the forecast period (2025-2033).
Enzyme replacement therapy (ERT) is a medical treatment used to replace missing or deficient enzymes in the body, typically for individuals with certain genetic disorders. These disorders often prevent the body from producing enough of a specific enzyme required for normal metabolic processes. ERT involves the administration of synthetic or recombinant versions of the deficient enzyme, either through intravenous (IV) infusion or other methods, to help manage symptoms and improve the quality of life for patients.
ERT is commonly used for treating lysosomal storage diseases, such as Gaucher disease, Fabry disease, and Pompe disease, among others. These diseases can cause the accumulation of substances in cells due to enzyme deficiencies, leading to various symptoms. By restoring the missing enzyme, ERT helps to reduce the buildup of these substances and alleviates symptoms.
The major factors propelling the enzyme replacement therapy market are rising advancements in biotechnology, regulatory support followed by rising product approvals, increasing investments in R&D, and growing awareness regarding rare genetic diseases with timely diagnosis and treatment.
- For instance, in March 2024, Collaborations Pharmaceuticals, Inc. was awarded a USD 3.9 million Small Business Innovation Research (SBIR) commercialization readiness pilot grant for Batten disease to manufacture intracerebroventricular enzyme replacement therapy.
Below Graph Represents the Number of Ert Products in the Process of Patenting in 2023 & 2024
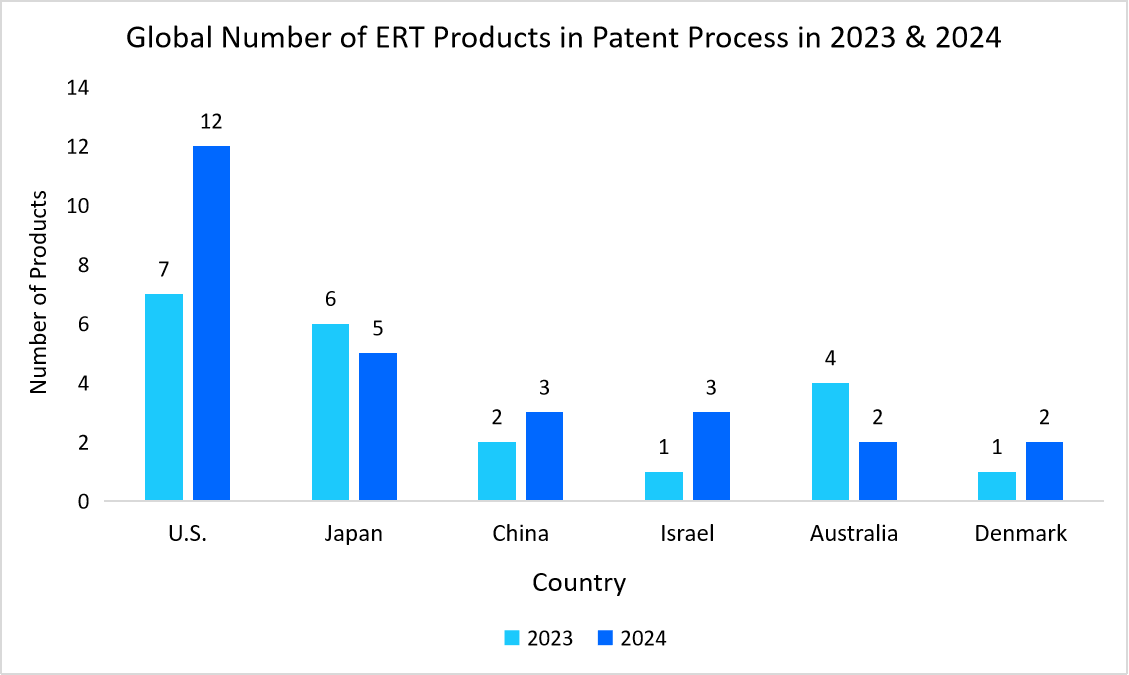
Source: World Intellectual Property Organization (WIPO) and Straits Research
Market Trends
Emergence of Recombinant Protein Technology Combined with Ert
The integration of recombinant protein technology with enzyme replacement therapy (ERT) is emerging as a key advancement in the treatment of rare genetic diseases. This innovative approach enables the design of specific proteins to address unmet medical needs, particularly in rare and complex disorders. Protalix, for instance, is advancing its enzyme replacement products using recombinant protein technology and protein enhancement modifications.
The company’s FDA-approved ProCellEx platform includes Elelyso, a novel ERT for type I Gaucher disease, and Elfabrio, an innovative treatment for Fabry disease. This combination of recombinant protein technology with ERT is significantly improving the treatment landscape for rare diseases, enhancing both efficacy and patient outcomes. As such, this trend is driving the global market's growth, offering promising solutions to conditions once untreatable.
Growing Trend of Enzymes Combined with Enzyme Stabilizers
A growing trend in the enzyme replacement therapy (ERT) market is the use of enzyme stabilizers alongside ERT to improve treatment outcomes. Some patients do not respond adequately to standard enzyme replacement therapies, posing challenges in disease management. However, combining ERT with enzyme stabilizers has proven to be more effective in such cases.
- For example, in September 2023, the U.S. Food and Drug Administration (FDA) approved a combination therapy of cipaglucosidase alfa (Pombiliti) and miglustat (Opfolda) for treating Pompe disease, a genetic disorder. This combination therapy enhances enzyme stability and improves overall treatment efficacy.
The success of such therapies is fueling market growth by providing effective, personalized solutions for difficult-to-treat diseases and addressing the needs of patients who previously had limited treatment options.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 10.12 Billion |
| Estimated 2025 Value | USD 10.95 Billion |
| Projected 2033 Value | USD 20.62 Billion |
| CAGR (2025-2033) | 8.23% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Sanofi, Takeda Pharmaceutical Company Limited, BioMarin, Amicus Therapeutics, Ultragenyx Pharmaceutical Inc. |

to learn more about this report Download Free Sample Report
Enzyme Replacement Therapy Market Growth Factors
Rising Regulatory Support
Increasing regulatory support, coupled with a growing number of product approvals, is a key driver of the global market. These approvals expand the availability of ERT products, thus offering more treatment options to patients with rare genetic disorders.
- For example, in 2023, the U.S. Food and Drug Administration (FDA) approved Lamzede (velmanase alfa) and ADZYNMA (ADAMTS13, recombinant-krhn), further validating the significance of regulatory backing in market growth. As more products receive approval, patients gain access to effective treatments, which enhances competition and innovation in the market.
This support not only facilitates the development of novel therapies but also boosts confidence in the ERT market, driving its expansion globally.
Rising Demand for Personalized Enzyme Therapy Approach
The rising demand for personalized enzyme therapy is increasingly shaping the ERT market. Personalized medicines tailored to an individual's genetic profile are gaining traction as they offer more targeted and effective treatments, especially for rare and complex diseases. Therefore, researchers are actively exploring personalized enzyme therapies, which aim to provide more precise treatments that align with a patient’s unique genetic makeup.
- A study published in the American Journal of Gastroenterology highlights the importance of incorporating genetic profiles to optimize pancreatic enzyme replacement therapy (PERT) for pancreatitis. This growing focus on personalized medicine is driving the ERT market by improving treatment efficacy, enhancing patient outcomes, and offering a more customized approach to care.
Restraining Factor
High Cost of Enzyme Replacement Therapy
The high cost of ERT is a significant restraint on market growth, limiting accessibility for many patients. The average annual cost of ERT can range from $40,941.1 to $69,176.5 per patient, placing a financial burden on both individuals and healthcare systems. This price barrier leads to challenges in insurance coverage, as many insurance providers struggle to cover the full cost of these treatments. The high cost also makes it difficult for patients in lower-income regions to access these therapies, hindering global market penetration. As a result, reducing the cost of ERT and improving reimbursement policies will be crucial for expanding patient access and driving broader market adoption.
Market Opportunity
Strong Product Pipeline
The robust product pipeline in the market presents a significant opportunity for growth. As ERT continues to emerge as a solution for treating rare genetic diseases, numerous products are advancing through clinical trials, offering hope for new treatment options.
- For example, M6P Therapeutics is currently developing two promising enzyme replacement therapies in its pipeline, i.e., GBA for Gaucher disease and GAA for Pompe disease. The development of these therapies is driving innovation and encouraging further research in the ERT field.
The introduction of new products will expand treatment options, enhance competition, and improve patient outcomes, thereby creating substantial opportunities for market growth and increased adoption of ERT globally.
Regional Insights
North America holds the largest revenue share in the ERT market, driven by several key factors. The region benefits from a well-established healthcare infrastructure, enabling efficient delivery of advanced therapies. Significant investments in research and development, along with a robust regulatory framework, have fostered the growth of innovative enzyme replacement products. Moreover, the presence of leading pharmaceutical companies like Sanofi, BioMarin, and Ultragenyx Pharmaceutical Inc. bolsters the market by ensuring continuous product advancements and accessibility.
U.s. Market Trends
The U.S. leads the enzyme replacement therapy (ERT) market thanks to continuous biotechnology research and increased funding for developing new ERT products. It holds the highest number of products in the patent process, with 13 in 2021, 15 in 2022, 7 in 2023, and 12 in 2024, as reported by the World Intellectual Property Organization (WIPO). This sustained innovation boosts the market's growth and positions the U.S. at the forefront.
Canada Market Trends
In Canada, government initiatives are driving the ERT market forward by improving access to drugs for rare diseases. A notable example is the bilateral agreement between the government and Saskatchewan to enhance drug access for patients with rare conditions. Such initiatives not only raise awareness about rare diseases but also improve treatment accessibility, creating a favorable environment for the market to thrive.
Asia-Pacific Enzyme Replacement Therapy Market Trends
Asia-Pacific is set to experience the fastest CAGR during the forecast period, driven by a combination of factors. Increasing awareness of rare genetic diseases and their associated therapies is fueling demand for enzyme replacement therapy (ERT). As the region’s healthcare sector continues to expand, investments in both public and private healthcare settings are accelerating, creating a favorable environment for ERT. Moreover, the growing pharmaceutical and biotechnology industries, coupled with enhanced regulatory frameworks, are contributing to the market’s growth.
India Market Trends
The Indian government’s commitment to treating rare diseases has significantly contributed to the growth of the enzyme replacement therapy industry. Financial support, such as the $23,102.29 allocated under the Rashtriya Arogaya Nidhi scheme in 2021, facilitates access to treatments across the country. These government initiatives help accelerate market growth by ensuring that patients with rare diseases receive the necessary support and access to specialized therapies like enzyme replacement treatments.
Europe Enzyme Replacement Therapy Market Trends
Germany Market Trends
Germany’s market is growing due to substantial investments from pharmaceutical companies focused on rare diseases. PTC Therapeutics is a prime example, pushing the envelope with enhanced patient advocacy and groundbreaking treatments for rare conditions. These continued investments and initiatives, aimed at improving awareness and care for rare disease patients, are key drivers of market expansion and foster innovative healthcare in Germany.
Uk Market Trends
In the UK, strategic investments in rare disease diagnosis and treatment are fueling the growth of the market. In July 2023, the UK government invested $17.10 million to enhance research on rare diseases, aiming to improve both diagnosis and treatment options. This boost in research funding is helping to develop new ERT products, thereby accelerating market growth and improving care for rare disease patients.
France Market Trends
France is witnessing significant growth in the market due to its high prevalence of rare diseases, with over 3 million people affected, representing 4.5% of the population. This large patient base creates a strong demand for disease-specific treatments like enzyme replacement therapy. The substantial number of rare disease cases drives market growth, as there is an urgent need for effective therapeutic solutions to address these conditions.
Product Insights
The agalsidase beta segment leads the ERT market due to its effectiveness in treating Fabry disease. This therapy stabilizes renal function, improves cardiac and gastrointestinal manifestations, and reduces the risk of cerebrovascular events. The segment's growth is also driven by strategic collaborations among companies to manufacture agalsidase beta. In February 2024, mAbxience and Biosidus signed a CDMO agreement to manufacture agalsidase beta, further expanding its availability and boosting market share.
Disease Type Insights
The mucopolysaccharidoses (MPS) segment holds the largest market share in the enzyme replacement therapy industry, driven by the high incidence of these diseases compared to others. MPS encompasses various types, including MPS I, II, III, IV, VI, and VII, with a combined prevalence of 1 in 20,000 to 25,000 live births in the U.S. This high incidence rate makes MPS a key focus for therapeutic development and drives demand for enzyme replacement therapies targeting these conditions.
Route of Administration Insights
The intravenous infusion segment dominates the enzyme replacement therapy industry due to the widespread availability of products in intravenous form. Intravenous infusion therapies are the primary treatment option for most enzyme replacement therapies, providing a reliable and efficient way to deliver therapeutic enzymes. In contrast, pancreatic ERT is the only category offering oral administration options, highlighting intravenous infusion as the most common and dominant method for enzyme therapy delivery.
End-User Insights
The hospital segment is the largest end-user in the market, as hospitals play a critical role in both outpatient and inpatient care. They provide comprehensive services, including pre-treatment evaluation, administration of therapies, and post-treatment monitoring. Hospitals are well-equipped to handle the complexities of enzyme replacement therapy, offering a structured environment for frequent visits and ongoing care, especially for patients with rare and complex diseases, making them a dominant force in the market.
Company Market Share
Key players in the global enzyme replacement therapy industry are actively pursuing strategic collaborations, acquisitions, and partnerships to strengthen their product portfolios and expand their market presence. These efforts allow companies to leverage each other’s expertise, resources, and technologies to develop more effective and targeted treatments for rare diseases.
Ultragenyx Pharmaceutical Inc.: An Emerging Provider in the Enzyme Replacement Therapy Market
Ultragenyx Pharmaceutical Inc. is an emerging player in the global market, recognized for its groundbreaking approach in gene therapy platforms and enzyme replacement treatments. The company focuses on developing novel therapies for rare and genetic diseases, aiming to address unmet medical needs.
Recent Development
- In May 2022, Ultragenyx Pharmaceutical Inc. acquired global rights to AAV gene therapy ABO-102 for Sanfilippo Syndrome Type A (MPS IIIA) from Abeona Therapeutics. This acquisition strengthens Ultragenyx’s position in the rare disease treatment market, expanding its gene therapy portfolio. ABO-102 is a promising therapy designed to treat the underlying cause of Sanfilippo Syndrome Type A, a devastating neurodegenerative disease.
List of Key and Emerging Players in Enzyme Replacement Therapy Market
- Sanofi
- Takeda Pharmaceutical Company Limited
- BioMarin
- Amicus Therapeutics
- Ultragenyx Pharmaceutical Inc.
- CHIESI Farmaceutici S.p.A.
- AstraZeneca
- Pfizer Inc.
- Protalix Biotherapeutics Inc.
- Leadiant Biosciences, Inc.
- Digestive Care, Inc.
- AbbVie Inc.
- Others
Recent Developments
- July 2024 – GC1130A, an experimental enzyme replacement therapy for Sanfilippo Syndrome Type A, was granted Fast Track status by the FDA. This designation comes after the agency approved a Phase 1 trial to test the therapy's safety. It follows previous orphan drug and rare pediatric disease designations, which aim to expedite its development.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 10.12 Billion |
| Market Size in 2025 | USD 10.95 Billion |
| Market Size in 2033 | USD 20.62 Billion |
| CAGR | 8.23% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Disease Type, By Route of Administration, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Enzyme Replacement Therapy Market Segments
By Product
- Imiglucerase
- Velaglucerase Alfa
- Pegademase
- Agalsidase Beta
- Laronidase
- Galsulfase
- Others
By Disease Type
- Gaucher Disease
- Pompe Disease
- Fabry Disease
- Mucopolysaccharidoses
- Lysosomal Acid Lipase Deficiency
- Others
By Route of Administration
- Intravenous Infusion
- Oral
By End-User
- Hospitals
- Clinics
- Home Care Setting
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































