Epinephrine Market Size, Share & Trends Analysis Report By Product (Autoinjectors, Pre-Filled Syringes, Others), By Route of Administration (Parenteral, Oral), By Application (Anaphylaxis, Cardiac Arrest, Respiratory Disorders, Others), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Epinephrine Market Size
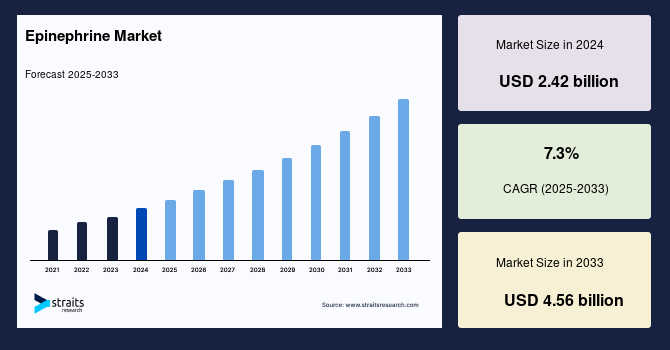
The global epinephrine market size was valued at USD 2.42 billion in 2024 and is projected to grow from USD 2.6 billion in 2025 to reach USD 4.56 billion by 2033, exhibiting a CAGR of 7.3% during the forecast period (2025-2033).
Epinephrine, also known as adrenaline, is a hormone and neurotransmitter naturally produced by the adrenal glands. It plays a key role in the body’s fight-or-flight response, preparing it for quick action in stressful or dangerous situations. When released, epinephrine increases heart rate, dilates airways, and raises blood sugar levels, providing the body with the energy and oxygen necessary for rapid movement.
The global epinephrine market is experiencing significant growth, driven by the rising prevalence of anaphylaxis, growing awareness of allergies, and advancements in drug delivery technologies. The increasing demand for user-friendly auto-injectors and needle-free epinephrine formulations, such as nasal sprays, is enhancing patient compliance and ease of administration. Government regulations, including mandates for stock epinephrine in schools and public spaces, are further propelling market growth.
- A notable example of this growth is the January 2025 launch of the "Neffy in School Program" by ARS Pharmaceuticals Inc., which provides eligible U.S. K-12 schools with free Neffy epinephrine nasal sprays. This initiative aims to improve access to life-saving treatments and enhance anaphylaxis preparedness in schools, contributing to the overall market expansion.
Moreover, the market is benefiting from increasing adoption in emergency medicine and expanding beyond its traditional applications, such as treating cardiac arrest and respiratory disorders. Strategic collaborations, ongoing investment in new drug formulations, and the expansion of retail pharmacy networks are opening up new opportunities.
Epinephrine Market Trends
Rising Awareness and Allergy Preparedness
As awareness of anaphylaxis management grows, more emphasis is being placed on the importance of early epinephrine administration. Educational campaigns, regulatory changes, and advocacy initiatives are expanding the use of epinephrine auto-injectors and nasal sprays across schools, workplaces, and public spaces.
- For example, in June 2023, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) clarified that spare adrenaline auto-injectors in schools could be used for anyone experiencing an unforeseen anaphylactic reaction, even without prior diagnosis or parental consent.
Such measures highlight the increasing focus on allergy preparedness, ensuring broader access to life-saving epinephrine in critical situations.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 2.42 Billion |
| Estimated 2025 Value | USD 2.6 Billion |
| Projected 2033 Value | USD 4.56 Billion |
| CAGR (2025-2033) | 7.3% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
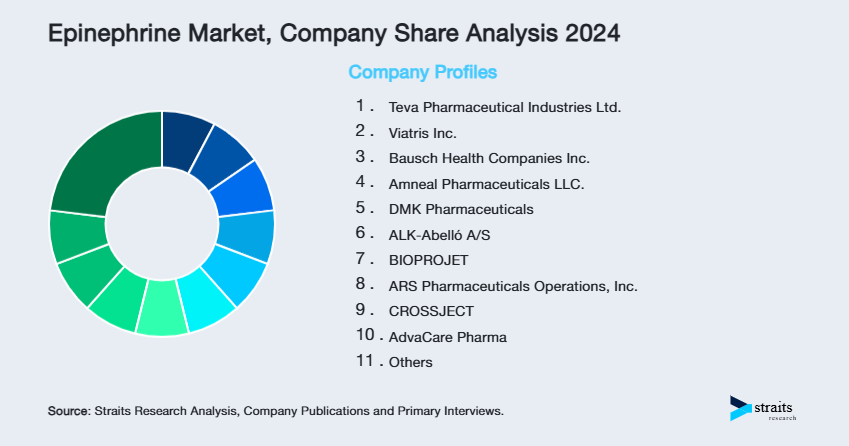
| Key Market Players | Teva Pharmaceutical Industries Ltd., Viatris Inc., Bausch Health Companies Inc., Amneal Pharmaceuticals LLC., DMK Pharmaceuticals |
to learn more about this report Download Free Sample Report
Epinephrine Market Growth Factors
Increasing Incidence of Anaphylaxis
The rising prevalence of food allergies, insect venom allergies, and drug-induced anaphylaxis is driving demand for the market for epinephrine. Changing dietary habits, environmental factors, and heightened allergen exposure contribute to a growing number of severe allergic reactions.
- For instance, in December 2023, a study published in the National Library of Medicine reported a wide variation in anaphylaxis incidence rates, ranging from 0.49 to 328.7 cases per 100,000 population per year. The data was derived from hospital admissions, emergency departments (EDs), multiple care providers, and epinephrine prescriptions.
The increasing prevalence highlights the growing burden of anaphylaxis, emphasizing the critical need for accessible and effective epinephrine treatment options.
Rising Government Initiatives
Governments worldwide are actively working to improve access to epinephrine through initiatives such as subsidized healthcare programs, school-based allergy awareness efforts, and public education campaigns. By emphasizing prevention, early intervention, and emergency preparedness, these initiatives play a crucial role in expanding epinephrine availability and usage, ultimately driving market growth.
- For example, in May 2023, Food Allergy Canada launched a series of initiatives aimed at enhancing access to epinephrine through advocacy, education, and public awareness campaigns. The organization focused on anaphylaxis preparedness, emphasizing the importance of stock epinephrine programs, which allow public institutions to store and administer unprescribed epinephrine auto-injectors for emergency use.
Such government and community-driven programs reinforce the importance of proactive allergy management, ensuring that epinephrine remains readily available in public spaces, workplaces, and educational institutions.
Market Restraining Factors
Lack of Proper Administration Awareness
A significant challenge in the global market is the lack of proper awareness and training regarding its administration. Many patients, caregivers, and even healthcare providers struggle with the correct injection techniques, leading to misuse or hesitation during emergencies. This lack of confidence in using epinephrine properly can reduce the effectiveness of the treatment and increase the risk of complications. Factors such as fear of self-injection, misinterpretation of allergic symptoms, and improper storage or handling of epinephrine auto-injectors contribute to underutilization. Addressing these issues through improved education, training programs, and public awareness initiatives is essential to ensure timely and effective use.
Market Opportunity
Development of Novel Formulations
Emerging drug delivery technologies, including needle-free nasal sprays, sublingual films, and microneedle patches, are being developed to enhance patient convenience, improve bioavailability, and minimize administration errors. These advancements address key limitations of traditional auto-injectors, making epinephrine treatment more accessible and user-friendly, especially in emergency situations.
- For example, in August 2024, ARS Pharmaceuticals, Inc. announced that the U.S. FDA approved Neffy, a 2 mg epinephrine nasal spray, for the treatment of Type I allergic reactions, including anaphylaxis. As the first needle-free epinephrine option, Neffy improves patient comfort and ease of administration, reducing barriers for individuals who may hesitate to use traditional injectors.
These groundbreaking innovations are broadening treatment choices, enhancing patient adherence, and unlocking new market opportunities, particularly for those who face challenges with conventional epinephrine delivery methods.
Regional Insights
North America: Dominant Region with 37.8% Market Share
North America leads the global epinephrine market, driven by a high prevalence of anaphylaxis, robust regulatory support, and the widespread adoption of autoinjectors. The region benefits from strong government initiatives that promote stock of epinephrine in schools and public spaces, ensuring quick access to life-saving treatments. Key market players, including Viatris Inc. and Pfizer Inc., play a pivotal role in market expansion through innovations and strategic partnerships. Continued efforts to raise awareness about anaphylaxis management further propel the region's dominance, solidifying its position.
Asia Pacific: Fastest Growing Region with the Highest Market Cagr
Asia-Pacific is anticipated to experience the highest CAGR due to a rising incidence of allergies and anaphylactic reactions, along with increasing public awareness and improved access to epinephrine products. Governments and healthcare organizations are prioritizing the development of anaphylaxis management programs while expanding retail pharmacy networks and improving product availability. Moreover, rapid urbanization, shifting dietary habits, and greater exposure to allergens are contributing to a surge in demand for epinephrine-based treatments, making this region a key growth area for the market.
Countries Insights
- U.S.- The U.S. epinephrine industry continues to lead, thanks to substantial investments in research and development by key pharmaceutical companies. Innovation is a primary driver, with companies introducing new and improved epinephrine products. In February 2025, Glenmark Pharmaceuticals launched a generic version of Epinephrine injection, gaining 180 days of competitive generic therapy (CGT) exclusivity. This move enhances accessibility and affordability, further solidifying the U.S. market as a critical player in epinephrine distribution.
- Germany- Germany stands as one of Europe's largest markets for epinephrine, with continued expansion fueled by increasing demand for epinephrine delivery devices. In October 2024, Ypsomed expanded its auto-injector production capacity by inaugurating a new manufacturing facility in Schwerin. This strategic move ensures the country meets the rising global demand for epinephrine products, positioning Germany as a key hub for innovation and production in Europe.
- France- France's market is growing due to strong government backing for innovative drug delivery solutions and a rising demand for needle-free alternatives. In July 2024, Crossject secured €6.9 million in funding to develop ZENEO Epinephrine, a needle-free prefilled delivery system. This investment, under the France 2030 Plan, aims to enhance patient comfort while expanding accessibility to epinephrine treatment, making France a key player in advancing epinephrine delivery technologies.
- India– India’s epinephrine market is experiencing rapid growth, fueled by a rising prevalence of allergies and increasing awareness of anaphylaxis. The Indian government's focus on improving healthcare infrastructure, combined with expanded pharmaceutical manufacturing, is driving market adoption. Moreover, the growing use of auto-injectors in hospitals and medical facilities across the country is contributing to the demand for effective and easily administered epinephrine treatments.
- China – China's epinephrine market is expanding rapidly, driven by a rising incidence of severe allergies and a growing awareness of anaphylaxis management. The country’s expanding healthcare infrastructure, coupled with government initiatives to improve emergency medical care, supports the market’s growth. Rising domestic pharmaceutical production and the increasing availability of cost-effective epinephrine treatment options further enhance access and adoption, driving China's market expansion.
Product Analysis
The autoinjectors segment leads the global market, generating the highest revenue due to their user-friendly design, portability, and ability to administer a rapid dose during emergencies. These devices provide pre-measured dosages, enabling patients to self-administer epinephrine quickly, reducing the risk of delays or errors. The convenience of autoinjectors, particularly in critical anaphylaxis situations, boosts patient compliance, contributing to their dominance in the market. This ease of use has made autoinjectors the go-to solution for emergency treatment.
Route of Administration Analysis
The parenteral route holds the largest share of the market, as injectable epinephrine is essential for managing severe allergic reactions, cardiac arrest, and respiratory emergencies. The quick onset of action and direct delivery into the bloodstream makes it the preferred option in life-threatening situations, ensuring fast and effective treatment. Parenteral administration provides more reliable outcomes in critical moments, particularly in hospitals and emergency settings, reinforcing its dominant position in the market.
Application Analysis
The anaphylaxis segment leads the market, driven by the rising prevalence of food allergies, insect venom allergies, and drug-induced anaphylaxis. With increasing public awareness and regulatory mandates for the availability of epinephrine in schools and public spaces, demand continues to grow. It represents one of the most common and life-threatening conditions requiring immediate epinephrine intervention, ensuring that this application remains a key market driver as awareness of its importance continues to spread.
Distribution Channel Insights
Hospital pharmacies dominate the market, driving the highest revenue due to their critical role in emergency care settings. These pharmacies ensure that injectable epinephrine is readily available in emergency departments and critical care units, which are pivotal during anaphylaxis, cardiac arrest, and respiratory emergencies. As hospital admissions due to these conditions continue to rise, the demand for epinephrine in hospital pharmacies remains strong, supporting the growth of the market. Their role in immediate treatment ensures timely access to life-saving medications.
Company Market Share
Key players in the global market are increasingly focusing on adopting critical business strategies such as strategic collaborations, product approvals, acquisitions, and product launches to enhance their market presence. By forming alliances with healthcare organizations and regulatory bodies, these companies can expand their reach and accelerate the development of innovative treatments.
Euroapi: An Emerging Player in the Global Epinephrine Market
EUROAPI is emerging as a significant player, leveraging its strong pharmaceutical manufacturing capabilities and strategic partnerships to drive growth. As a leading European API (active pharmaceutical ingredients) manufacturer, EUROAPI focuses on producing high-quality, cost-effective epinephrine formulations. By expanding its portfolio and adopting innovative drug delivery technologies, such as auto-injectors and needle-free solutions, the company aims to meet the growing demand for epinephrine treatments globally.
List of Key and Emerging Players in Epinephrine Market
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Bausch Health Companies Inc.
- Amneal Pharmaceuticals LLC.
- DMK Pharmaceuticals
- ALK-Abelló A/S
- BIOPROJET
- ARS Pharmaceuticals Operations, Inc.
- CROSSJECT
- AdvaCare Pharma
- Cambrex Corporation
- Endo, Inc
- Pfizer Inc
- Farbe Firma Pvt. Ltd
- EUROAPI
to learn more about this report Download Market Share
Recent Developments
- November 2024 –ALK-Abelló signed a USD 145 million licensing deal with ARS Pharmaceuticals for exclusive global rights to neffy, the first approved needle-free epinephrine nasal spray. This strategic move strengthens ALK-Abelló’s presence in anaphylaxis treatment, reinforcing its long-term commitment to allergy care.
- November 2024 – Endo launched ADRENALIN Ready-to-Use Premixed Bag, the first and only FDA-approved, manufacturer-prepared epinephrine premixed IV bag. This innovation streamlines emergency treatment by eliminating the need for manual preparation, reducing the risk of dosing errors, and expediting administration in critical settings such as ICUs, emergency rooms, and surgical procedures.
Analyst Opinion
As per our analyst, the global epinephrine market is poised for substantial growth, driven by the rising prevalence of anaphylaxis, the increasing incidence of severe allergic reactions, and growing awareness of emergency allergy management. The market is witnessing rapid innovation, with advancements such as needle-free nasal sprays, auto-injectors featuring improved usability, and extended shelf-life formulations, all of which are enhancing accessibility, convenience, and patient compliance.
Asia-Pacific presents a particularly lucrative opportunity, supported by improving healthcare infrastructure, rising disposable incomes, and growing awareness of anaphylaxis management. However, despite these positive trends, the market faces notable challenges. The high cost of branded epinephrine auto-injectors, stringent regulatory requirements, and limited awareness in underdeveloped regions continue to hinder widespread adoption.
Moreover, concerns related to product recalls, supply chain disruptions, and pricing controversies remain key barriers. Nevertheless, ongoing R&D efforts, strategic partnerships, and the emergence of generic alternatives are expected to mitigate these challenges, ensuring sustained market expansion in the coming years.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 2.42 Billion |
| Market Size in 2025 | USD 2.6 Billion |
| Market Size in 2033 | USD 4.56 Billion |
| CAGR | 7.3% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Route of Administration, By Application, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Epinephrine Market Segments
By Product
- Autoinjectors
- Pre-Filled Syringes
- Others
By Route of Administration
- Parenteral
- Oral
By Application
- Anaphylaxis
- Cardiac Arrest
- Respiratory Disorders
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































