Extractable and Leachable Testing Services Market Size, Share & Trends Analysis Report By Product Type (Container Closure Systems, Single-use Systems, Drug Delivery Systems, Others), By Application (Parenteral Drug Products, Orally Inhaled and Nasal Drug Products, Ophthalmic) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Extractable and Leachable Testing Services Market Overview
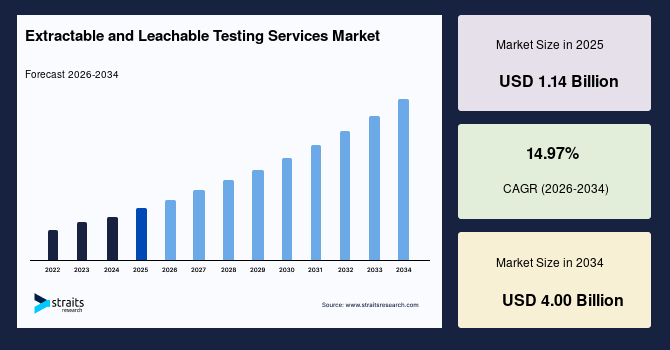
The global extractable and leachable testing services market size is estimated at USD 1.14 billion in 2025 and is projected to reach USD 4.00 billion by 2034, growing at a CAGR of 14.97% during the forecast period. Remarkable growth of the market is propelled by the rising adoption of high-performance analytical instruments for polymer characterisation and the increasing regulatory emphasis on material compatibility in biologics and medical device packaging.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 40.47% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 16.98%.
- Based on Product Type, the container closure systems segment dominated the market in 2025, accounting for 34.71% of share in 2025.
- Based on Application, the orally inhaled and nasal drug products segment dominated the market in 2025 with a revenue share of 44.23%.
- The U.S. dominates the global market, valued at USD 373.74 million in 2024 and reaching USD 428.08 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
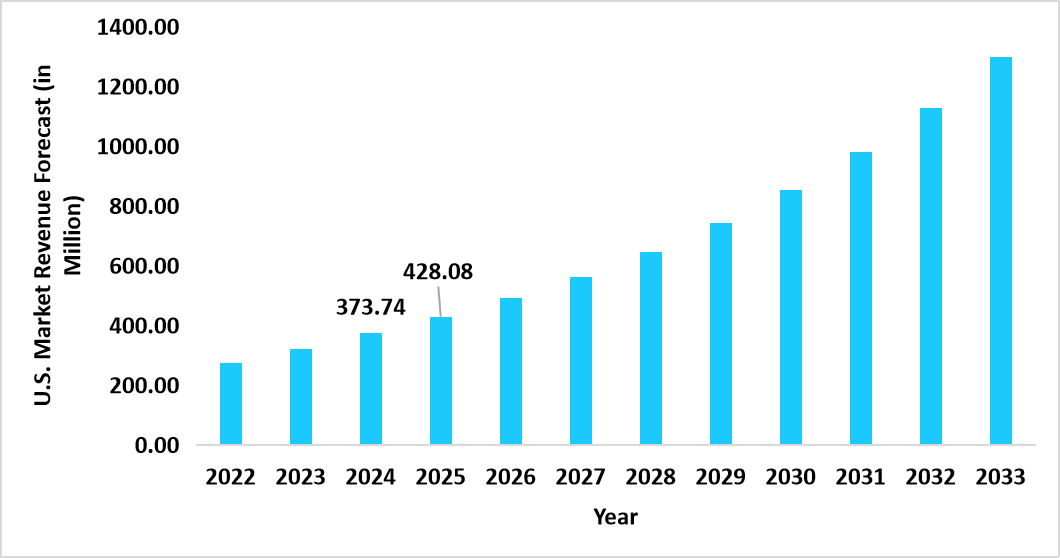
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.14 billion
- 2034 Projected Market Size: USD 4.00 billion
- CAGR (2025 to 2034): 14.97%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The extractable and leachable testing services market encompasses analytical evaluations conducted to identify and quantify chemical substances that may migrate from packaging materials, manufacturing components, or delivery systems into pharmaceutical and biopharmaceutical products. These services are essential to ensure product safety, stability, and compliance with global regulatory guidelines throughout the product lifecycle. Based on product type, the market is segmented into container closure systems, which include vials, ampoules, syringes, and cartridges evaluated for potential extractable compounds; single-use systems, covering disposable bioprocessing equipment and polymer-based assemblies used in biologics manufacturing; drug delivery systems, such as inhalers, prefilled syringes, and transdermal devices tested for leachable migration; and others, comprising laboratory consumables and diagnostic containers that require compatibility verification. By application, the market is categorized into parenteral drug products, focusing on injectable formulations sensitive to packaging interactions; orally inhaled and nasal drug products, which require stringent control of leachable due to direct respiratory exposure; and ophthalmic products, where testing ensures the chemical purity and material compatibility of containers used for eye-care formulations. Together, these segments represent a comprehensive analytical framework that supports regulatory compliance and safeguards pharmaceutical product integrity across diverse therapeutic applications.
Latest Market Trends
Integration of Computational Modelling with Experimental E&L Testing
A key trend in the extractables and leachable (E&L) testing services market is the integration of computational modelling with laboratory-based extraction studies. Analytical service providers are incorporating simulation-driven approaches to predict potential leachable compounds before experimental validation. This combined method enables targeted testing, reduces time spent on non-critical compound screening, and enhances the precision of risk assessment for complex pharmaceutical and medical device packaging systems. The use of predictive algorithms is also supporting regulatory submissions by providing mechanistic insights into material–drug interactions across varying conditions.
Expansion of Digital Quality Management Systems in Analytical Laboratories
The emerging trend is the adoption of digital quality management platforms in E&L testing laboratories to streamline documentation and compliance tracking. Laboratories are increasingly transitioning from paper-based records to electronic data management systems that ensure traceability of analytical procedures, instrument calibration, and result reporting. This digital shift enhances workflow transparency, facilitates audit readiness, and aligns with global data integrity requirements set by regulatory authorities. It also supports faster project turnaround and better coordination among global laboratory networks handling multi-site studies.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.14 Billion |
| Estimated 2026 Value | USD 1.31 Billion |
| Projected 2034 Value | USD 4.00 Billion |
| CAGR (2026-2034) | 14.97% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Pace, Smithers , Auriga Research Private Limited, SCHOTT Pharma AG & Co. KGaA , Analytica Chemie |
to learn more about this report Download Free Sample Report
Market Drivers
Increasing Complexity of Biologic and Combination Products
The growing development of biologics, cell-based therapies, and combination drug-device products is driving the demand for E&L testing services. These advanced formulations are highly sensitive to packaging components, which require detailed extractables profiling and toxicological evaluation to prevent contamination. Regulatory authorities are mandating comprehensive leachable assessments during early-stage development, prompting pharmaceutical companies to partner with specialized laboratories. The need to ensure chemical compatibility, maintain product stability, and meet evolving global safety standards is reinforcing market growth.
Market Restraints
High Analytical Cost and Lengthy Method Validation Processes
The Extractable and Leachable Testing Services Market faces challenges due to high operational costs associated with advanced instrumentation and extensive validation requirements. Complex analytical workflows involving LC–MS, GC–MS, and HRMS demand skilled personnel, costly reagents, and long testing timelines. These factors limit participation by smaller laboratories and delay project completion for clients. Additionally, variations in regional regulatory expectations create procedural redundancies, further increasing testing expenses and reducing overall efficiency.
Market Opportunities
Emergence of E&L Testing in Nutraceutical and Regenerative Medicine Packaging
The growing application of extractables and leachable testing services beyond pharmaceuticals presents a new opportunity for market expansion. Nutraceutical manufacturers and regenerative medicine developers are increasingly assessing packaging and container interactions to ensure product safety and compliance with evolving quality standards. This shift is encouraging analytical service providers to diversify their portfolios and develop specialised methods tailored to non-traditional product categories. The entry of these new industries into regulatory-driven quality testing frameworks is expected to generate additional demand for E&L services globally.
Regional Analysis
North America held the largest share of 40.47% in the extractable and leachable testing services market in 2025, supported by the increasing regulatory scrutiny from agencies such as the U.S. FDA and Health Canada regarding packaging materials, parenteral drugs, and medical devices. The region’s established network of analytical laboratories and contract research organizations continues to drive demand for qualified testing and toxicological risk assessments in compliance with USP <1663> and <1664>. The growing outsourcing of analytical testing by pharmaceutical and biotechnology firms is contributing to the expansion of full-service testing providers.
The U.S. market is characterized by strong adoption of E&L testing across biologics, combination products, and single-use systems. Pharmaceutical companies are increasingly partnering with accredited laboratories to conduct extractables profiling and leachable quantification in line with FDA and PQRI guidelines. The integration of LC–MS and GC–MS platforms with digital data management systems is enhancing trace compound identification, supporting better regulatory submissions and manufacturing oversight.
Asia Pacific Market Insights
The Asia Pacific region is projected to register the fastest growth during the forecast period, driven by the expansion of biologics manufacturing and the establishment of regional GMP-compliant testing facilities. Regulatory harmonization across countries such as Japan, China, and South Korea is encouraging standardized analytical validation procedures for packaging compatibility studies. Rising investments by pharmaceutical and contract research firms are strengthening the local presence of extractable and leachable testing laboratories.
The Indian extractable and leachable testing services market is developing rapidly with increased demand from domestic pharmaceutical exporters complying with U.S. and European packaging standards. Public–private partnerships are promoting the establishment of specialized analytical laboratories equipped with advanced spectrometric technologies. Integration of laboratory information management systems under national digital health frameworks is enhancing traceability of analytical results and improving coordination between drug developers and regulatory authorities.
Pie Chart: Regional Market Share, 2025
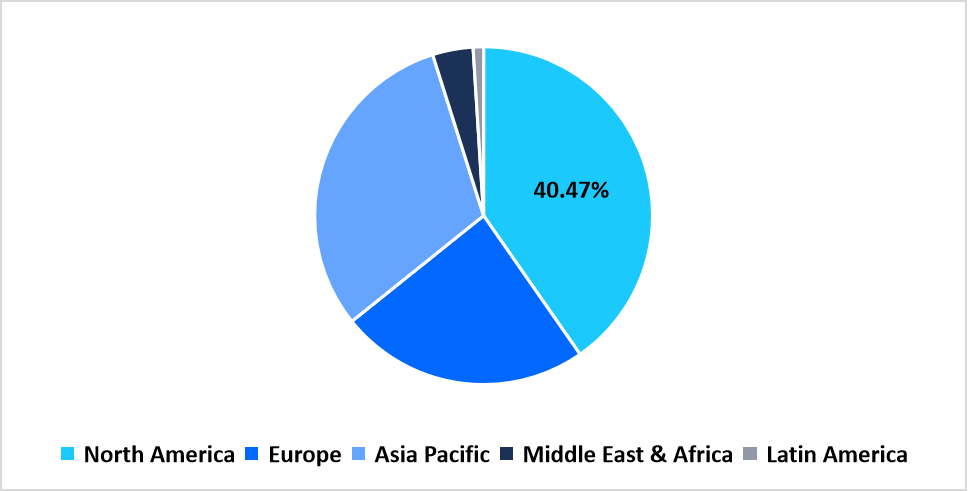
Source: Straits Research
Europe Market Insights
Europe’s market is expanding with the growing enforcement of EMA guidelines on container closure integrity and material compatibility for parenteral formulations. Pharmaceutical manufacturers are increasingly conducting detailed migration and leachable studies during early formulation development. Collaboration between national reference laboratories and regulatory agencies is reinforcing method standardisation across member states, contributing to improved quality assurance within the supply chain.
Germany’s market growth is driven by advancements in analytical instrumentation and the presence of certified contract labs offering multi-technique E&L workflows. Local pharmaceutical and medical device firms are adopting comprehensive leachable evaluations aligned with the EU Medical Device Regulation, promoting safer packaging and product stability.
Middle East and Africa Market Insights
The Middle East and Africa region is witnessing expansion due to ongoing investment in pharmaceutical manufacturing zones and the establishment of regional analytical testing partnerships. Increasing regulatory alignment with international pharmacopeial standards is encouraging manufacturers to perform detailed extractables studies on imported packaging materials. Academic-industrial collaborations are enhancing local expertise in analytical toxicology, supporting future regulatory independence.
The UAE market is expanding with the Ministry of Health promoting compliance testing for pharmaceutical packaging and sterile devices. Hospitals and diagnostic laboratories are integrating centralised testing systems for extractable assessments in parenteral formulations, strengthening overall product safety evaluation.
Latin America Market Insights
The Latin America market is growing as regional pharmaceutical production scales up and governments emphasise packaging safety and drug stability testing. Adoption of international testing standards is advancing through cooperation between national regulatory agencies and global testing providers. Capacity-building initiatives are enhancing analytical quality management within local laboratories.
Brazil’s market growth is supported by national programs encouraging analytical capability enhancement within public and private testing institutions. Collaborations with multinational CROs are increasing access to validated extractables and leachable testing procedures for domestic pharmaceutical and medical device manufacturers, enabling compliance with ANVISA and international guidelines.
Product Type Insights
The container closure systems segment dominated the market in 2025, accounting for 34.71% of share in 2025. The dominance is attributed to the extensive use of vials, syringes, cartridges, and ampoules in pharmaceutical packaging, which require detailed extractables and leachable assessments to ensure product integrity. Stringent regulatory expectations for parenteral drugs and biologics have increased the frequency of testing for glass, elastomeric, and plastic components used in these systems.
The single-use systems segment is projected to record the fastest growth of 15.34% during the forecast period, due to the widespread transition toward disposable bioprocessing equipment in biologics manufacturing. The rise in adoption of polymer-based materials for filtration and storage in single-use assemblies is increasing the demand for chemical compatibility studies and leachable profiling. Continuous scaling of biologics production and the growing focus on contamination control across biomanufacturing facilities are accelerating segment growth.
Application Insights
The orally inhaled and nasal drug products segment dominated the market in 2025 with a revenue share of 44.23%. The dominance is driven by the higher analytical requirements for propellants, valves, and polymeric components used in metered-dose inhalers and nasal sprays. Pharmaceutical companies are prioritizing leachable evaluation to comply with stringent inhalation product safety standards set by regulatory agencies, ensuring patient safety and consistent dose delivery.
The parenteral drug products segment is projected to witness the fastest growth, registering a CAGR of 15.23% over the forecast period. The increase in production of injectable biologics, vaccines, and sterile formulations has intensified the need for extractables profiling of container closure and delivery systems. Ongoing research into packaging interactions and the growing focus on minimizing extractable migration into sensitive formulations are fostering continuous demand for specialized analytical testing.
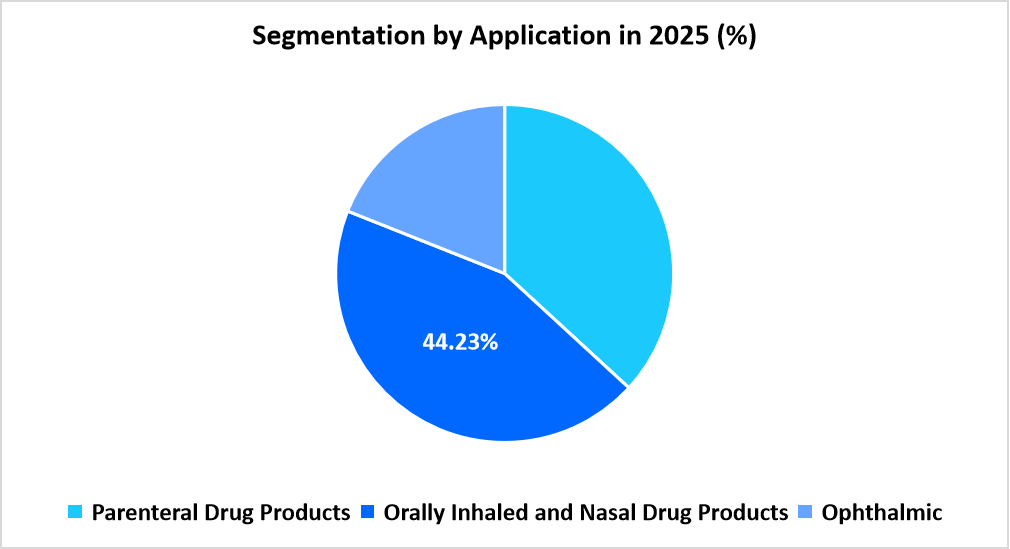
Source: Straits Research
Competitive Landscape
The global Extractable and Leachable Testing Services Market was moderately consolidated, with participation from established analytical service providers, contract research organisations (CROs), and emerging specialised laboratories focusing on material characterisation and regulatory compliance.
Auriga Research Private Limited: An emerging market player
Auriga Research Private Limited emerged as a notable player in the Extractable and Leachable Testing Services Market, offering specialized studies to evaluate the impact of packaging materials, container-closure systems, and medical devices on drug product safety and stability. In 2024, Auriga Research expanded its analytical testing portfolio by introducing dedicated extractable and leachable (E&L) study services. The offering included identification and quantification of leachables using GC–MS and LC–MS techniques, alongside risk assessment and regulatory documentation support compliant with USP <1663>/<1664> and FDA guidance. This initiative reinforced Auriga’s role as an emerging service provider in pharmaceutical and medical device testing within the Asia-Pacific region, demonstrating its growing commitment to regulatory-compliant analytical excellence.
List of Key and Emerging Players in Extractable and Leachable Testing Services Market
- Pace
- Smithers
- Auriga Research Private Limited
- SCHOTT Pharma AG & Co. KGaA
- Analytica Chemie
- Gateway Analytical
- Reading Scientific Services Ltd
- Eurofins Scientific
- Intertek Group plc
- SGS Société Générale de Surveillance SA
- WuXi AppTec
- Merck KGaA
- West Pharmaceutical Services, Inc.
- Pacific Biolabs
- BA Sciences
- Sotera Health Academy
- Charles River Laboratories
- Labcorp
- Others
Strategic Initiatives
- October 2024: Recipharm AB announced that it had expanded its pharmaceutical‐development capabilities through strategic investments and the integration of advanced technologies. The company aimed to offer services for early- and late-stage product development, including clinical-trial supply and commercial technologies.
- February 2024: Thermo Fisher Scientific, Inc. expanded its service portfolio by adding mycoplasma and other biosafety-testing capabilities at its GMP site in Middleton (Wisconsin). The laboratory also provided biologics testing, small-molecule analysis, medical-device functionality testing, and extractables & leachables testing.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.14 Billion |
| Market Size in 2026 | USD 1.31 Billion |
| Market Size in 2034 | USD 4.00 Billion |
| CAGR | 14.97% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Application |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Extractable and Leachable Testing Services Market Segments
By Product Type
- Container Closure Systems
- Single-use Systems
- Drug Delivery Systems
- Others
By Application
- Parenteral Drug Products
- Orally Inhaled and Nasal Drug Products
- Ophthalmic
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































