Fluorescent In Situ Hybridization Probe Market Size, Share & Trends Analysis Report By Type (DNA Probes, RNA Probes), By Technology (Quantitative FISH, Multiplex FISH, Conventional FISH), By Application (Cancer Diagnostics, Genetic Diseases, Cytogenetics, Other), By End User (Research, Clinical Use, Companion Diagnostics) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Fluorescent In Situ Hybridization Probe Market Overview
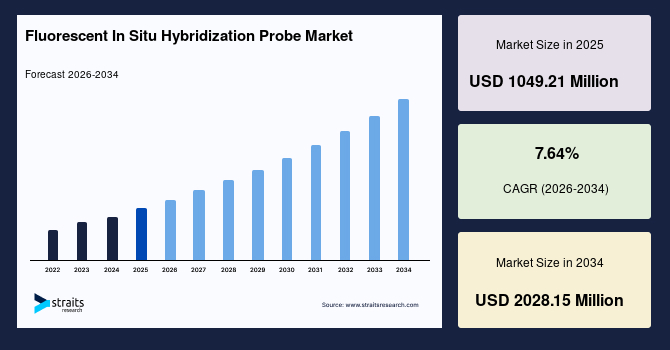
The global fluorescent in situ hybridization probe market size is estimated at USD 1049.21 million in 2025 and is projected to reach USD 2028.15 million by 2034, growing at a CAGR of 7.64% during the forecast period. The remarkable growth of the market is due to the rising prevalence of genetic disorders and cancer, increasing adoption of personalized medicine, and advancements in molecular diagnostics. The American Cancer Society reported that in 2025, over 2 million new cancer cases are expected in the U.S., excluding non-melanoma skin cancers, which illustrates the large and growing oncology patient pool. Such a factor depicted the critical role of FISH probes in enabling precise genetic testing, guiding therapy-linked decisions, and improving overall patient outcomes in cancer and genetic disorder management.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 47.23% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 8.56%.
- Based on Type, the RNA probes segment is expected to register the fastest CAGR growth of 8.97%.
- Based on Technology, the quantitative FISH segment dominated the market in 2025 with a revenue share of 36.73%.
- Based on the Application, the cancer diagnostics segment dominated the market in 2025, with a revenue share of 44.25%.
- Based on End User, the companion diagnostics segment is expected to register the fastest CAGR growth of 9.07%.
- The U.S. dominates the global market, valued at USD 399.35 million in 2024 and reaching USD 428.15 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)

Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1049.21 million
- 2034 Projected Market Size: USD 2028.15 million
- CAGR (2025 to 2034): 7.64%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The global fluorescent in situ hybridization (fish) probe market encompasses a diverse range of molecular diagnostic tools designed to identify and visualize specific DNA or RNA sequences within cells and tissues, supporting accurate disease diagnosis and research applications. By type, the market is segmented into DNA probes, widely utilized for detecting chromosomal abnormalities and gene amplifications in cancer and genetic studies, and RNA probes, primarily used for analysing gene expression and detecting RNA viruses, offering high sensitivity and specificity. By technology, the market includes quantitative FISH, enabling precise measurement of gene copy numbers, multiplex FISH, allowing simultaneous detection of multiple genetic targets for complex analyses, and conventional FISH, extensively used in cytogenetic laboratories for standard chromosomal mapping and visualization. By application, FISH probes find broad use in cancer diagnostics, genetic disease detection, and cytogenetics, as well as in other fields such as microbial identification and prenatal testing. By end user, the market serves research laboratories, clinical diagnostic centers, and companion diagnostics applications, underscoring its expanding role in precision medicine, translational research, and targeted therapy development. Collectively, advancements in probe labeling, imaging systems, and automation continue to enhance the accuracy, throughput, and clinical utility of the FISH probe market worldwide.
Latest Market Trends
Shift from General Diagnostics to Therapy-Linked Companion Diagnostics
A major trend in the fluorescent in situ hybridization probe market is the shift from using FISH probes mainly for general cancer diagnostics to employing them as companion diagnostics directly tied to specific therapies. As per the U.S. FDA, the FDA had approved a PMA supplement for the Vysis ALK Break Apart FISH Probe Kit to include an indication for ENSACOVE (ensartinib) in non-small cell lung cancer (NSCLC), linking FISH testing directly to treatment decision making. This development highlighted the move from broad diagnostic use towards therapy-linked precision testing, which not only improved clinical adoption of FISH probes but also strengthened their role in guiding targeted cancer treatments.
Shift from High Regulatory Barriers to Streamlined Approvals
A key trend in the global market is the shift from highly restrictive regulatory requirements to a more streamlined approval pathway. For example, in June 2025, the U.S. FDA reclassified FISH-based devices for detecting chromosomal abnormalities from the more restrictive Class III to Class II (special controls), which simplified regulatory requirements and facilitated faster clinical adoption. This change reflected the move from lengthy, complex approval processes to a more efficient regulatory pathway, which reduced barriers for manufacturers, accelerated product approvals, and supported faster market availability of advanced FISH probe products.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1049.21 Million |
| Estimated 2026 Value | USD 1125.59 Million |
| Projected 2034 Value | USD 2028.15 Million |
| CAGR (2026-2034) | 7.64% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Hoffmann-La Roche Ltd, Agilent Technologies, Inc., Abbott, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc. |
to learn more about this report Download Free Sample Report
Market Drivers
Technological Advancements in the FISH Technique
A key driver in the FISH probe market is the rapid improvement in technologies, such as multiplex FISH, which make probes more specific, faster and more sensitive. For example, in March 2024, Oxford Gene Technology IP Limited’s Cytocell launched its latest multiplex FISH panel capable of simultaneously detecting multiple chromosomal abnormalities in a single assay, which further reduced processing time while improving sensitivity and specificity.
Market Restraint
High Cost Associated with FISH Assays
A major restraint in the FISH probe market is the high cost associated with the FISH assays, which restricts their adoption across clinical settings. This is especially challenging for complex tissue analyses, where advanced probe design and specialized handling are required. As a result, the expensive nature of these assays slowed widespread implementation, limited market growth, and accessibility of FISH-based diagnostics.
Market Opportunity
Growing Use of Fluorescent In Situ Hybridization (FISH) Probe Market in Hematologic Malignancies
An opportunity in the FISH probe market is its expanding use for hematologic cancers, where chromosomal abnormalities play a critical role in diagnosis and treatment selection. Recently, Empire Genomics and BioDot launched hematology-focused FISH probe panels for multiple myeloma, validated on the BioDot CellWriter S platform. This reflected a strong opportunity for companies to develop disease-specific panels that enhance diagnostic efficiency and automation.
Regional Analysis
The North America region dominated the market with a revenue share of 47.23% in 2025. The growth is attributed to factors such as high adoption of advanced cytogenetic technologies in cancer care, funding from the National Institutes of Health (NIH) for genetic and oncology research, and early regulatory approvals by the U.S. Food and Drug Administration (FDA) for advanced FISH-based diagnostic assays.
The fluorescent in situ hybridization probe market in the U.S. is widely driven by the FDA approvals of FISH companion diagnostics. These approvals make FISH testing mandatory for selecting targeted therapies, which directly increases adoption and expands market size. This regulatory backing ensures sustained demand, fueling consistent market growth and strengthening overall global market growth nationwide.
Asia Pacific Market Insights
The Asia Pacific region is the fastest-growing region with a CAGR of 8.56% during the forecast timeframe. The growth is attributed to factors such as the adoption of advanced molecular diagnostic techniques, such as FISH, as well as growing awareness about early disease detection. These factors improve access and boost the market size and market growth.
The fluorescent in situ hybridization probe market in China is fuelled by the government's inclusion of FISH-based tests in official clinical guidelines and insurance reimbursement. MOH guidelines in China now require FISH tests for certain blood cancers like myelodysplastic syndromes, CLL, and CML, and a few provincial insurance programs cover the cost of approved FISH probes. These policies raise diagnostic use, expanding market size, and boosting market growth.
Pie Chart: Regional Market Share, 2025

Source: Straits Research
Europe Market Insights
In Europe, the fluorescent in situ hybridization probe market is driven by the strict reimbursement policies that cover FISH testing for non-small cell lung cancer, ensuring wider patient access. Along with this, initiatives, such as the Network Genomic Medicine (NGM) program, strengthen molecular diagnostics, boosting demand, expanding market size, and supporting sustainable fluorescent in situ hybridization probe market growth.
The fluorescent in situ hybridization probe market in the UK is growing due to the NHS Genomic Medicine Service, which includes FISH testing in the National Genomic Test Directory. In addition, government funding through the Genome UK strategy, expansion of regional genomic laboratories, and NHS partnerships with leading diagnostic companies are increasing accessibility. These efforts boost early cancer detection, expand market size, and ensure sustainable fluorescent in situ hybridization probe market growth.
Middle East and Africa Market Insights
The market is growing due to enhanced research and development funding by governments and other organizations, which is fostering the development and adoption of advanced FISH technologies in the region.
The market is growing in South Africa due to increasing private healthcare investments and the expansion of molecular diagnostic laboratories. Several diagnostic chains in Gauteng province started integrating FISH technology to offer faster and more accurate cancer and genetic testing services, boosting regional market adoption.
Latin America Market Insights
The market is growing in Latin America due to increasing collaborations between local research institutions and international biotech companies, which are expanding access to advanced FISH technologies. Joint initiatives in Argentina and Chile facilitated the transfer of FISH-based diagnostic platforms to regional hospitals and laboratories, accelerating market growth.
The market in Argentina is growing due to increasing private sector investment in biotechnology and molecular diagnostics. Several private laboratories in Buenos Aires offered FISH-based cancer and prenatal testing services, expanding access to advanced diagnostics and boosting market demand.
Type Insights
The RNA probes segment is anticipated to grow at a CAGR of 8.97% during 2026-2034. The growth is attributed to the increasing adoption of RNA-based FISH technologies such as single-molecule RNA FISH (smFISH) and multiplex RNA FISH, which enable precise visualization of gene expression at the cellular level.
The DNA probes segment dominated the market in 2025, owing to the widespread adoption of DNA-based assays for detecting chromosomal abnormalities and gene rearrangements, along with their higher specificity, stability, and compatibility with a wide range of diagnostic applications.
Technology Insights
The quantitative FISH segment is expected to dominate the market in 2025 with a revenue share of 36.73%. This growth is attributed to the increasing demand for accurate measurement of telomere length, gene amplification, and chromosomal copy number variations in both clinical diagnostics and research applications.
The multiplex FISH segment is anticipated to register the fastest CAGR of 8.23% during the forecast period, owing to its ability to improve diagnostic efficiency, reduce turnaround time, and enable comprehensive genomic profiling in cancer and genetic research.

Source: Straits Research Analysis
Application Insights
The cancer diagnostics segment dominated the market in 2025, with a revenue share of 44.25%. The growth is attributed to the rising global cancer burden, increasing use of FISH probes for detecting chromosomal abnormalities and gene rearrangements, and the growing preference for companion diagnostics in targeted cancer therapies.
The genetic diseases segment is anticipated to register the fastest CAGR of 8.56% during the forecast period, driven by the rising prevalence of hereditary disorders and growing utilization of FISH assays for prenatal and postnatal genetic screening.
End User Insights
The clinical use segment dominated the market in 2025, accounting for a revenue share of 46.22%, owing to the widespread implementation of FISH testing in hospitals and diagnostic laboratories for accurate disease diagnosis and patient management.
The companion diagnostics segment is expected to register the fastest CAGR growth of 9.07%, owing to the rising use of FISH-based assays for therapy selection, and growing collaborations between diagnostic developers and pharmaceutical companies to co-develop targeted treatment solutions.
Competitive Landscape
The global fluorescent in situ hybridization probe market is highly fragmented, with numerous established players and emerging companies competing across various regions.
Cynvenio Biosystems, Inc.: An emerging market player
Cynvenio Biosystems, Inc., is an emerging player in the fluorescent in situ hybridization probe market, specializing in FISH-based liquid biopsy solutions for detecting chromosomal abnormalities in circulating tumor cells.
- In March 2025, Cynvenio Biosystems, Inc. announced the launch of its CLEAR Liquid Biopsy FISH Panel, designed to detect multiple chromosomal abnormalities in circulating tumor cells.
List of Key and Emerging Players in Fluorescent In Situ Hybridization Probe Market
- Hoffmann-La Roche Ltd
- Agilent Technologies, Inc.
- Abbott
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc.
- Genemed Biotechnologies, Inc.
- Oxford Gene Technology IP Limited
- Biosearch Technologies
- Merck KGaA
- QIAGEN
- PerkinElmer, Inc.
- BioDot
- Abnova Corporation
- Biocare Medical, LLC.
- Daicel Arbor Biosciences
- Precision Medicine Group, LLC
- Cell Culture Company, LLC
- Advanced Cell Diagnostics, Inc.
- BioGenex.
- Leica Biosystems Nussloch GmbH
- Others
Strategic Initiatives
- July 2025: PerkinElmer Inc. introduced the CellWriter S platform, a high-throughput imaging system designed to streamline fluorescence in situ hybridization (FISH) workflows.
- April 2025: Empire Genomics partnered with BioDot to launch the first pre-optimized hematology FISH probe panels and controls for the CellWriter S platform.
- January 2025: Hoffmann-La Roche Ltd’s PATHWAY HER2 (4B5) test received FDA approval as the first HER2 ultralow companion diagnostic for metastatic breast cancer, expanding trastuzumab deruxtecan eligibility and demonstrating strong concordance with HER2 FISH assays.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1049.21 Million |
| Market Size in 2026 | USD 1125.59 Million |
| Market Size in 2034 | USD 2028.15 Million |
| CAGR | 7.64% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Technology, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Fluorescent In Situ Hybridization Probe Market Segments
By Type
- DNA Probes
- RNA Probes
By Technology
- Quantitative FISH
- Multiplex FISH
- Conventional FISH
By Application
- Cancer Diagnostics
- Genetic Diseases
- Cytogenetics
- Other
By End User
- Research
- Clinical Use
- Companion Diagnostics
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































