Genetic Toxicology Testing Market Size, Share & Trends Analysis Report By Product (Reagents & Consumables, Assays Kit, Services), By Type (In Vitro, In Vivo), By Assay (Comet Assay, Micronucleus Assay, Chromosomal Aberration Test, Genetic Mutation Test, Others), By Application (Pharmaceutical & Biotechnology, Food Industry, Cosmetics Industry, Other) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Genetic Toxicology Testing Market Overview
The global genetic toxicology testing market size is estimated at USD 1.57 billion in 2025 and is projected to reach USD 4.10 billion by 2034, growing at a CAGR of 11.28% during the forecast period. Remarkable growth of the market is propelled by the rising demand for early-stage safety screening of new chemical entities across pharmaceutical, food, and consumer product industries.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 39.13% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.28%.
- Based on Product, the services segment dominated the market with a revenue share of 47.83% in 2025.
- Based on Type, the in vivo segment registered the fastest growth with 12.43% during the forecast period.
- Based on the Assay, the comet assay segment dominated the market with a 41.23% share in 2025.
- Based on Application, the pharmaceutical and biotechnology segment dominated the market with a 49.53% share in 2025.
- The U.S. dominates the global genetic toxicology testing market, valued at USD 502.03 million in 2024 and reaching USD 556.50 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
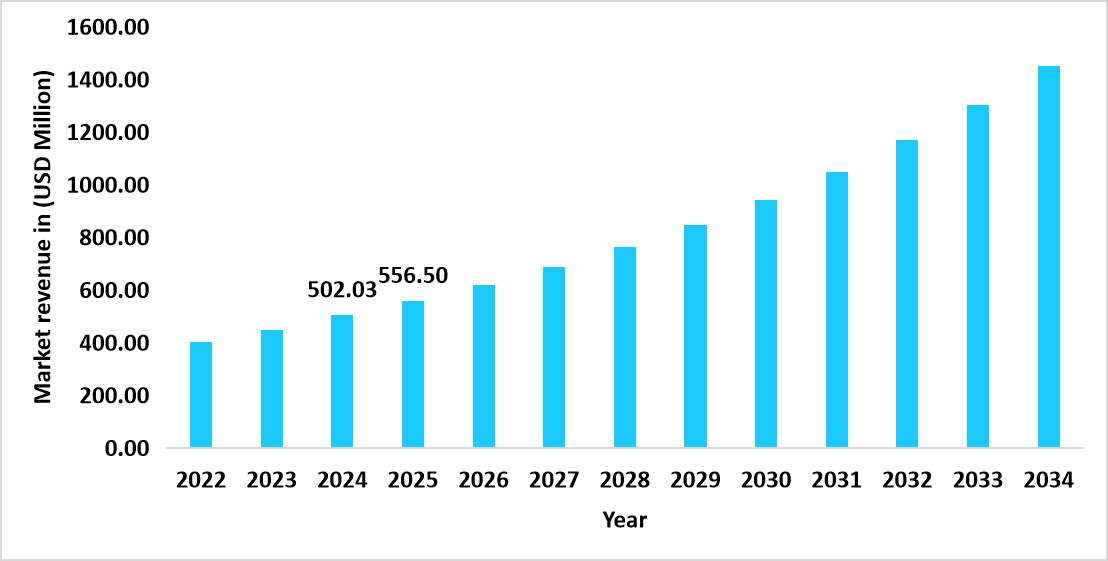
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.57 billion
- 2034 Projected Market Size: USD 4.10 billion
- CAGR (2025 to 2034): 11.28%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The genetic toxicology testing market comprises a broad range of products, assays, and service models used to evaluate the potential of chemicals, pharmaceuticals, consumer products, and environmental substances to induce genetic damage. The market includes reagents and consumables, assay kits, and services that support routine and specialized testing workflows across laboratory environments. Testing is conducted through both in vitro and in vivo approaches to assess DNA strand breaks, chromosomal alterations, mutation events, and other genetic endpoints using methods such as comet assays, micronucleus assays, chromosomal aberration tests, genetic mutation tests, and related analytical techniques. These solutions are widely adopted by pharmaceutical and biotechnology companies for preclinical safety studies, by the food industry to evaluate additives and processing by-products, by the cosmetics industry for ingredient screening, and by other sectors that require structured assessment of genotoxic potential to meet regulatory and safety standards.
Latest Market Trends
Growing Use of High Content Imaging in Genotoxicity Screening
Laboratories are increasingly adopting high-content imaging platforms to observe cellular responses during genotoxicity assays. These systems allow detailed visualization of nuclear changes, chromatin alterations, and early stress responses during DNA damage studies. This trend is expanding interest in image-based genotoxicity workflows that provide richer datasets compared to traditional endpoint readings.
Rising Application of Human Stem Cell-Derived Models in Genetic Toxicology
Research facilities are introducing human stem cell-derived cultures into genotoxicity studies to capture cellular responses that align more closely with human physiology. These models are being used to examine DNA damage, mutation formation, and chromosomal alterations in specialized tissues, creating new avenues for complex assay development across pharmaceutical and chemical safety programs.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.57 billion |
| Estimated 2026 Value | USD 1.74 billion |
| Projected 2034 Value | USD 4.10 billion |
| CAGR (2026-2034) | 11.28%% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific Inc., Charles River Laboratories International, Inc., Eurofins Scientific, SGS Société Générale de Surveillance SA, Inotiv |
to learn more about this report Download Free Sample Report
Genetic Toxicology Testing Market Driver
Growing Emphasis on Mechanistic Genotoxicity Evaluation in Early Discovery
Drug discovery and chemical safety programs are placing a stronger focus on understanding mechanisms behind DNA damage pathways rather than relying solely on basic mutation endpoints. This shift encourages the adoption of assays that measure oxidative stress responses, DNA repair activity, and pathway-specific biomarkers. As a result, organizations performing early hazard screening are integrating a broader range of genetic toxicology tools to support deeper interpretation of molecular events.
Market Restraint
Limited Standardization Across Global Laboratories for Emerging Assays
The market faces a restraint due to variations in protocols, instrumentation, and scoring criteria for newer genotoxicity assay formats. Differences across laboratories slow harmonization efforts and reduce consistency in study outcomes. This lack of alignment creates challenges for organizations that seek uniform datasets for regulatory or comparative testing purposes.
Market Opportunity
Expansion of Artificial Intelligence-Based Prediction Models for Genotoxicity Risk
There is a rising opportunity for AI-driven platforms that analyze chemical structures, biological pathways, and historical assay data to predict genotoxicity risk prior to laboratory testing. These tools are attracting interest from pharmaceutical, agrochemical, and consumer product companies aiming to streamline early selection of safer compounds. Wider integration of AI-based models is expected to support faster screening and broaden the adoption of computational toxicology within the field.
Regional Analysis
North America held the largest share of 39.13% in the genetic toxicology testing market in 2025 due to the extensive adoption of preclinical safety assessment programs across pharmaceutical and biotechnology companies. The region benefits from a strong ecosystem of CROs and toxicology laboratories that conduct mutagenicity, cytogenetic, and chromosomal damage studies aligned with regulatory submission requirements. Growing interest in early hazard detection for small molecules and biologics continues to reinforce market expansion across commercial and academic facilities.
The U.S. market grows due to rising preclinical pipelines in oncology, metabolic disorders, and rare diseases, which have increased the use of assays such as Ames tests, micronucleus assays, and in vitro chromosomal studies. Toxicology divisions across U.S.-based drug developers expanded their outsourcing volume to meet accelerated study timelines for investigational programs. A new wave of facility upgrades in private CROs supported higher throughput studies, further boosting demand for genetic toxicology services.
Asia Pacific Market Insights
Asia Pacific emerged as the fastest-growing region with a 13.28% CAGR due to rising investments in toxicology infrastructure and increased participation in international drug development programs. Research centers across the region continue to expand assay capabilities for mutagenic and chromosomal damage studies as regulatory guidelines in emerging economies become more structured. Expanding collaboration between domestic institutes and global sponsors has accelerated the adoption of genetic toxicology testing tools across early discovery and regulatory pathways.
China experienced strong growth driven by government-supported expansion of preclinical research parks and rising capability building within national laboratories. New toxicology centers in major cities introduced upgraded in vitro genotoxicity services to support chemical, agrochemical, and pharmaceutical submissions. Participation in multinational development programs further increased the usage of chromosomal aberration and mutation screening assays across private and public sector institutions.
Pie Chart: Regional Market Share, 2025
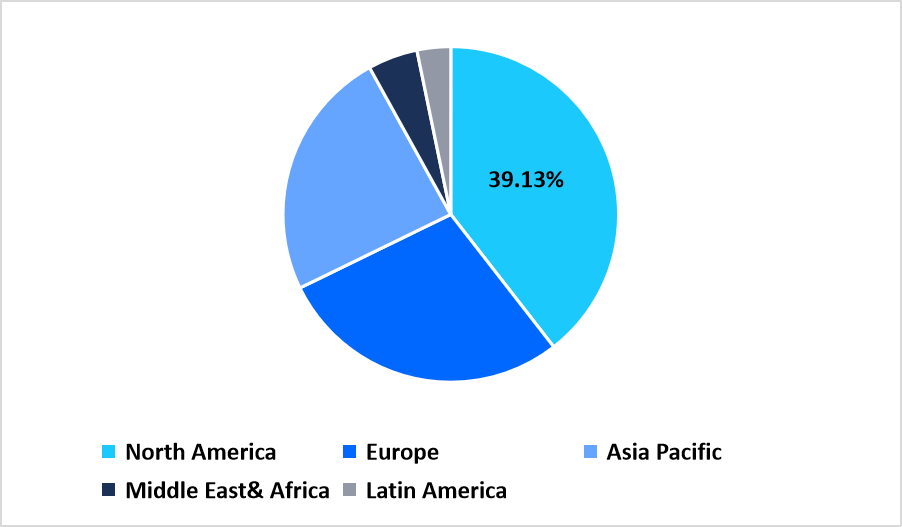
Source: Straits Research
Europe Market Insights
Europe recorded steady growth due to regulatory requirements under REACH and evolving standards for safety assessment across chemicals, pharmaceuticals, and consumer products. These frameworks encouraged laboratories and CROs to expand assay coverage and adopt standardized study designs across member states. Expansion of toxicology research consortia also influenced the wider use of genetic test panels for risk evaluation across diverse product classes.
The UK market advanced as research organizations and service providers broadened their portfolio of genotoxicity assays to support both early discovery and late-stage regulatory testing. Adoption of integrated study workflows in contract laboratories grew as sponsors sought consolidated reporting formats for regulatory submissions. A rise in chemical and pharmaceutical safety programs within academic facilities further added to assay demand.
Middle East and Africa Market Insights
The Middle East and Africa region showed growth driven by the rising establishment of toxicology laboratories within new research hospitals and university science clusters. Government-backed initiatives aimed at strengthening chemical safety monitoring and environmental risk assessment contributed to the introduction of genotoxicity testing capabilities in select national facilities.
In South Africa, growth was influenced by expanding research activity in environmental toxicology and occupational exposure assessment. Public sector laboratories increased their focus on mutagenicity studies for industrial chemicals and pollutants. Universities in major cities integrated additional coursework and laboratory programs focused on genetic toxicology, creating broader adoption of core assays within academic settings.
Latin America Market Insights
Latin America continued to develop its genetic toxicology testing capacity due to the modernization of laboratory infrastructure and rising interest in regulatory compliance for pharmaceuticals, agrochemicals, and cosmetic ingredients. Regional governments introduced programs aimed at expanding toxicology capabilities within public laboratories, supporting increased participation in global safety testing networks.
Brazil expanded its market presence as large research institutions strengthened toxicology departments to support the safety evaluation of new chemical entities and food ingredients. Growing involvement in contract studies commissioned by global sponsors encouraged local laboratories to scale up mutagenicity and chromosomal assays. Enhanced training initiatives for laboratory personnel contributed to stronger adoption of structured testing workflows.
Product Insights
The services segment dominated the market with a revenue share of 47.83% in 2025. This dominance is due to high demand from pharmaceutical, biotechnology, chemical, and regulatory-driven safety programs that rely on outsourced facilities to conduct assays such as micronucleus, chromosomal aberration, and comet tests. CROs and specialized toxicology laboratories continue to expand capacity and assay availability, which supports greater utilization of service-based offerings.
The reagents and consumables segment recorded the fastest growth with 12.12%, driven by rising assay volumes in academic, industrial, and contract laboratories that require a consistent supply of assay kits, staining reagents, buffers, and culture media. Growth in routine mutation screening and cytogenetic evaluations across diverse product categories continues to increase consumption of consumables in genetic toxicology workflows.
Type Insights
The in vitro segment dominated the market in 2025, reflecting its wide adoption across research and regulatory settings for early hazard identification. Laboratories prefer in vitro methods for preliminary screening of pharmaceuticals, food ingredients, agrochemicals, and cosmetic formulations due to structured workflows and suitability for high-throughput testing.
The in vivo segment registered the fastest growth with 12.43%, driven by rising requirements for confirmatory chromosomal and mutation studies that support regulatory submissions. Growth in complex chemical evaluations and late-stage pharmaceutical toxicology programs continues to expand the use of in vivo protocols.
Assay Insights
The comet assay segment dominated the market with a 41.23% share in 2025. Its wide usage in DNA strand break assessment across environmental studies, pharmaceutical safety programs, and industrial chemical screening has increased demand for laboratories offering this assay. Its flexibility across tissue types and exposure models further strengthens its adoption.
The genetic mutation test segment grew the fastest at 12.45%, supported by increasing use of mutation detection methods for early screening of new chemical entities and consumer product ingredients. Rising interest in detecting point mutations and gene-level alterations has contributed to higher test volumes in this segment.
Application Insights
The pharmaceutical and biotechnology segment dominated the market with a 49.53% share in 2025. Expansion of preclinical research pipelines, rising submissions for small molecules and biologics, and continuous regulatory oversight have increased reliance on genotoxicity testing across drug development stages. Companies consistently require structured assay panels to meet global regulatory guidelines, supporting large-scale test utilization.
The food industry segment experienced the fastest growth with 12.78%, driven by rising assessments of food additives, packaging-related chemicals, and processing by-products for potential genetic toxicity. Regulatory agencies across regions continue to encourage structured evaluation of food-related substances, which supports the adoption of screening assays within this segment.
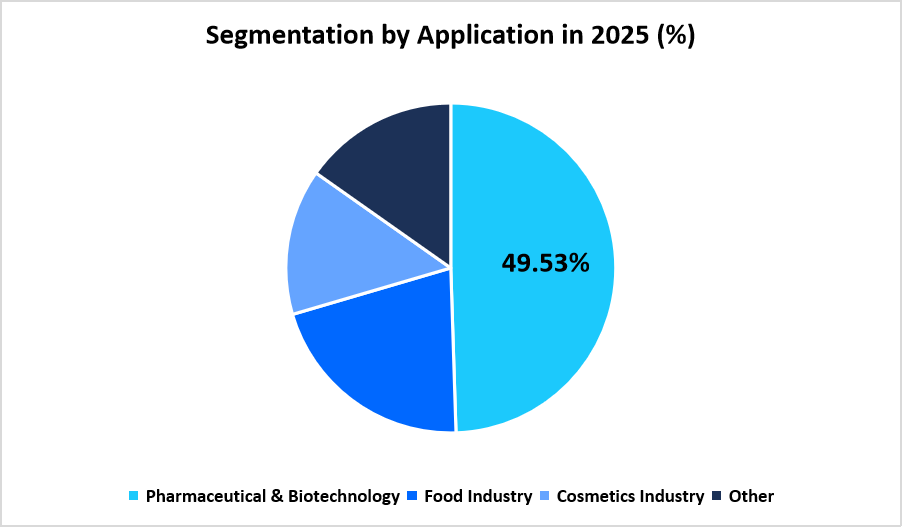
Source: Straits Research
Competitive Landscape
The global genetic toxicology testing market is moderately fragmented with a mix of full-service contract research organizations, specialist toxicology laboratories, and large diagnostics and testing conglomerates offering assay panels, in vitro and in vivo testing services, and supporting reagents and instrumentation.
Charles River Laboratories International, Inc.: An emerging market player
Charles River Laboratories International, Inc. provides a broad suite of genetic toxicology assays across in vitro and in vivo models, along with study design and regulatory consulting for pharmaceutical and biotech clients. In recent years, the company expanded its preclinical safety assessment capabilities to support integrated genotoxicity testing programs for small molecules and biologics.
List of Key and Emerging Players in Genetic Toxicology Testing Market
- Thermo Fisher Scientific Inc.
- Charles River Laboratories International, Inc.
- Eurofins Scientific
- SGS Société Générale de Surveillance SA
- Inotiv
- MB Research Laboratories
- Gentronix
- Syngene International Limited
- Creative Bioarray
- Toxys
- XENOMETRIX AG
- KOREA INSTITUTE OF TOXICOLOGY
- Nelson Laboratories, LLC
- CompareNetworks, Inc.
- Others
Strategic Initiatives
- July 2025: Thermo Fisher Scientific, Inc. received FDA approval for its Oncomine Dx Express Test, enabling rapid next-generation sequencing–based tumor profiling via NGS solutions.
- September 2024: Scantox Group acquired Gentronix Ltd, expanding its genetic toxicology testing portfolio and enhancing global capacity for preclinical and genotoxicity testing services across the pharmaceutical and biotech sectors.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.57 billion |
| Market Size in 2026 | USD 1.74 billion |
| Market Size in 2034 | USD 4.10 billion |
| CAGR | 11.28%% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Type, By Assay, By Application |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Genetic Toxicology Testing Market Segments
By Product
- Reagents & Consumables
- Assays Kit
- Services
By Type
- In Vitro
- In Vivo
By Assay
- Comet Assay
- Micronucleus Assay
- Chromosomal Aberration Test
- Genetic Mutation Test
- Others
By Application
- Pharmaceutical & Biotechnology
- Food Industry
- Cosmetics Industry
- Other
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































