Hemostasis Diagnostics Market Size, Share & Trends Analysis Report By Product (Laboratory Analyzers), By Test (Activated Partial Thromboplastin Time, D-Dimer Test, Fibrinogen Test, Prothrombin Time (PT) Test, Other Tests), By End User (Hospitals, Diagnostic Laboratories, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Hemostasis Diagnostics Market Overview
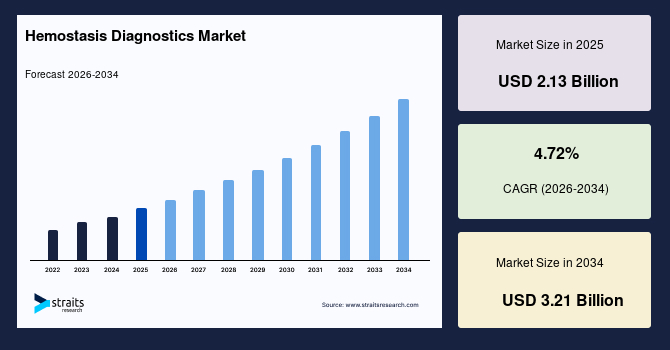
The global hemostasis diagnostics market size is estimated at USD 2.13 billion in 2025 and is projected to reach USD 3.21 billion by 2034, growing at a CAGR of 4.72% during the forecast period. The remarkable growth of the market is due to the increasing prevalence of bleeding and thrombotic disorders, as well as the rising demand for rapid and precise coagulation testing.
Key Market Trends & Insights
- North America held a dominant share of the global market with a market share of 38.43% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 6.68%.
- Based on the Product, the reagents & consumables segment dominated the market with a revenue share of 65.24% in 2025.
- Based on the Test, the prothrombin time (PT) test segment accounted for the largest revenue share of 33.45% in 2025.
- Based on End Users, the hospitals segment dominated the market in 2025, with a revenue share of 61.23%
- The U.S. dominates the hemostasis diagnostics market, valued at USD 734.11 million in 2024 and reaching USD 765.60 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
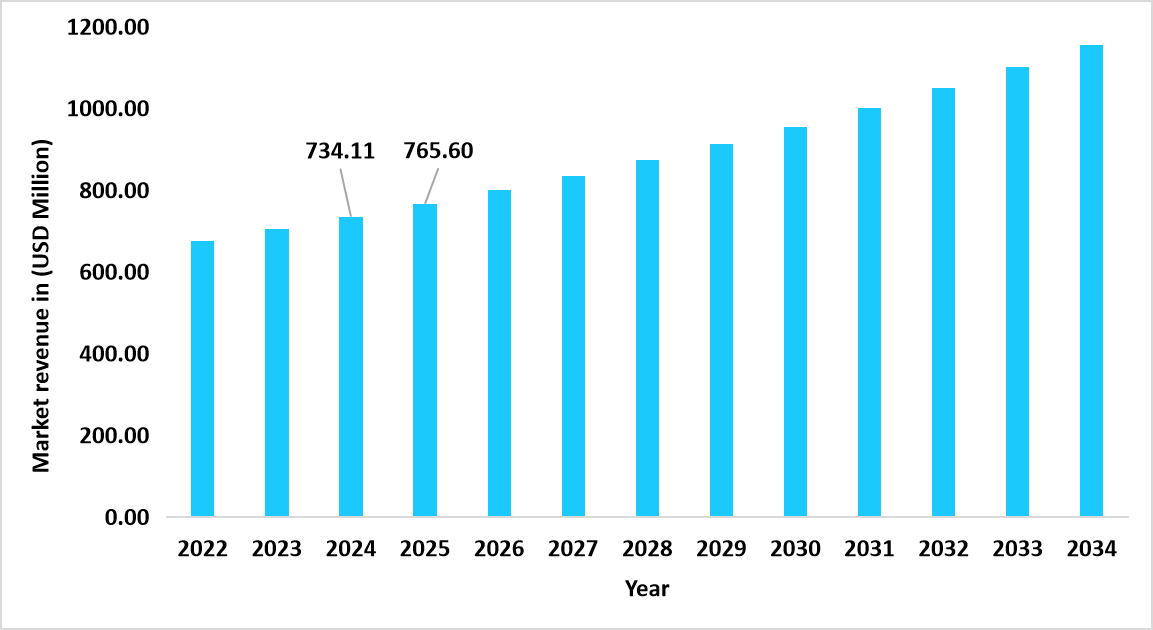
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.13 billion
- 2034 Projected Market Size: USD 3.21 billion
- CAGR (2025 to 2034): 4.72%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The hemostasis diagnostics market refers to the industry focused on technologies and solutions used to assess, monitor, and manage blood coagulation processes to diagnose bleeding and thrombotic disorders. It includes laboratory analyzers (automated, semi-automated, and manual systems) that deliver precise, high-throughput testing; point-of-care testing systems that enable rapid, near-patient assessment; and reagents and consumables such as assay kits, calibrators, and controls that support routine and specialized coagulation analyses.
Based on test type, the market comprises Activated Partial Thromboplastin Time (APTT), D-Dimer, Fibrinogen, Prothrombin Time (PT), and other coagulation assays that evaluate different pathways of Hemostasis. By end user, the market serves hospitals, diagnostic laboratories, and other healthcare facilities, which rely on these diagnostic tools for surgical preparation, anticoagulant therapy monitoring, and critical care decision-making. Overall, the market plays a crucial role in advancing clinical accuracy, improving patient outcomes, and supporting early detection and personalized management of coagulation-related disorders.
Market Trends
Shift from Conventional Coagulation Testing to AI-Driven and Integrated Diagnostic Ecosystems
The hemostasis diagnostics market is undergoing a major transformation marked by a shift from manual, parameter-based coagulation testing to integrated, AI-powered and data-driven diagnostic ecosystems. Laboratories and hospitals are increasingly adopting artificial intelligence and advanced analytics to interpret coagulation data, detect assay irregularities, and predict thrombotic or bleeding risk with higher precision. Automated analyzers integrated with cloud-based platforms enable continuous quality control, workflow optimization, and real-time clinical decision support. This evolution from standalone tests to intelligent diagnostic networks is improving diagnostic accuracy, reducing operator dependency, and enhancing the overall efficiency of coagulation management.
Shift from Centralized Laboratory Testing to Decentralized Point-of-Care and Personalized Coagulation Management
The emerging trend is the movement from centralized laboratory-based coagulation testing to decentralized, patient-centered point-of-care (POC) and personalized testing models. Advances in portable coagulometers, cartridge-based systems, and connected handheld analyzers are enabling rapid, near-patient results in emergency, surgical, and outpatient settings. This decentralization supports immediate clinical decision-making, especially for anticoagulant therapy monitoring and critical care scenarios. The growing integration of wireless connectivity and remote result interpretation further promotes personalized hemostasis management, empowering clinicians to tailor anticoagulant dosing and improve patient safety outcomes.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.13 Billion |
| Estimated 2026 Value | USD 2.22 Billion |
| Projected 2034 Value | USD 3.21 Billion |
| CAGR (2026-2034) | 4.72% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Abbott, Danaher Corporation, Hoffmann-La Roche Ltd, Grifols, S.A., Werfen |
to learn more about this report Download Free Sample Report
Market Drivers
Rising Burden of Bleeding and Thrombotic Disorders Coupled with Advancements in Coagulation Testing Technologies
The growing global incidence of cardiovascular diseases, liver disorders, and genetic bleeding conditions is driving demand for accurate coagulation assessment. The introduction of next-generation analyzers with high throughput, improved sensitivity, and connectivity capabilities has enhanced diagnostic capacity. Innovations such as optical clot detection, cartridge-based microfluidic assays, and high-stability reagents have made testing faster, more reproducible, and less labour-intensive. Furthermore, increased use of coagulation monitoring in surgical and critical care settings is reinforcing the clinical importance of hemostasis diagnostics in patient safety and treatment optimization.
Market Restraints
High Operational Costs and Limited Access to Advanced Testing in Low-Resource Settings
Despite technological progress, the high cost associated with automated analyzers, proprietary reagents, and continuous calibration remains a challenge for smaller laboratories and public healthcare facilities. Limited infrastructure for laboratory automation and the shortage of skilled laboratory professionals further restrict the adoption in emerging economies. Additionally, frequent maintenance requirements and dependency on imported consumables increase operational costs. These financial and infrastructural constraints impede uniform market penetration, especially in rural and underserved regions where basic coagulation screening remains underutilized.
Market Opportunity
Emergence of Personalized Hemostasis Management and Digital Connectivity Solutions
The growing focus on personalized medicine presents a major opportunity for the hemostasis diagnostics market. Integration of patient specific factors such as genetic polymorphisms, drug response profiles, and comorbidity data enables precision-based coagulation monitoring. Digital connectivity between analyzers, electronic health records (EHRs), and telepathology platforms allows real-time result interpretation and remote decision support, improving patient outcomes. The expanding role of home-based coagulation monitoring and wearable biosensors for chronic anticoagulant users further widens market potential. This convergence of digital health and individualized diagnostics is expected to transform coagulation testing from reactive measurement to proactive management.
Regional Analysis
North America dominated the hemostasis diagnostics market in 2025 with a share of 38.43%, driven by well-established healthcare infrastructure, strong reimbursement systems, and early adoption of advanced coagulation analyzers and automated testing platforms. The region’s focus on precision medicine and clinical laboratory automation continues to enhance diagnostic accuracy and reduce turnaround time. Increasing demand for rapid and point-of-care coagulation testing in emergency and surgical settings is further accelerating growth.
The U.S. market expansion is fueled by the integration of high-throughput hemostasis analyzers within hospital networks and independent diagnostic laboratories. The Centers for Medicare & Medicaid Services (CMS) initiatives supported laboratory modernization and quality-based reimbursements that have encouraged investments in innovative coagulation testing systems.
Asia-Pacific Market Insights
Asia-Pacific is the fastest-growing regional market, exhibiting a strong compound annual growth rate of 6.68% due to expanding hospital laboratories, government-led diagnostic capacity building, and increasing healthcare expenditure. Rising awareness of coagulation disorders, coupled with the growing adoption of point-of-care testing devices in urban and rural healthcare centers, is boosting market penetration.
India’s market growth is driven by the expansion of private diagnostic chains, government-funded health screening programs, and the increasing availability of low-cost hemostasis testing devices. The National Health Mission’s initiatives to improve early diagnosis of bleeding disorders and the surge in demand for pre-surgical coagulation assessments are expanding the clinical application scope for hemostasis diagnostics in both urban and semi-urban regions.
Pie Chart: Regional Market Share, 2025
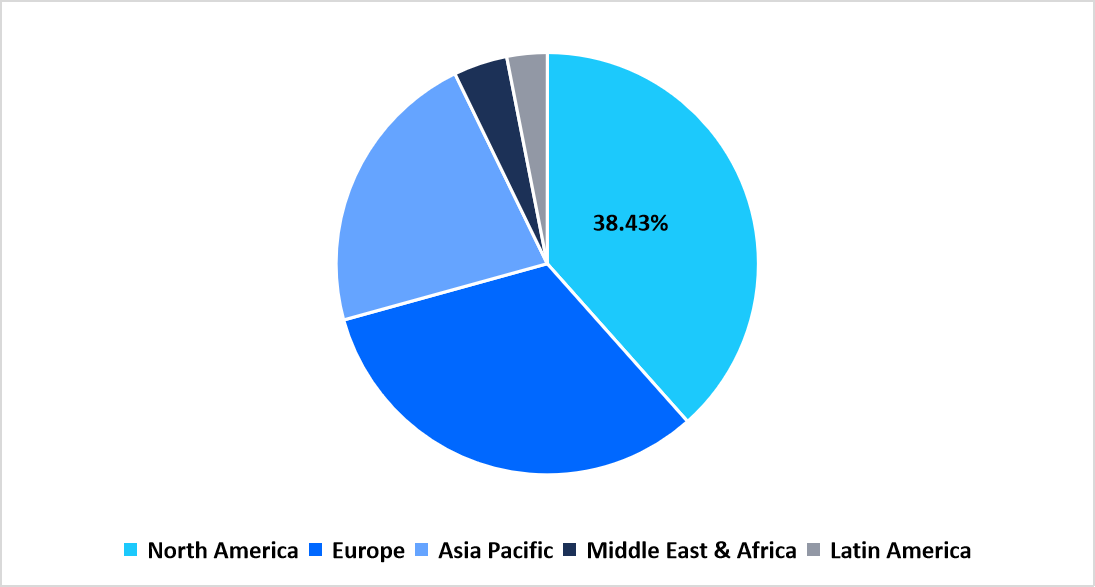
Source: Straits Research
Europe Market Insights
Supported by well-defined clinical guidelines for coagulation disorder management and widespread implementation of laboratory automation, enhances the market growth. The region’s emphasis on quality assurance and participation in international external quality assessment (EQA) programs has elevated testing reliability across healthcare facilities. Continuous clinical research in thrombosis and anticoagulant therapy monitoring contributes to steady demand for advanced diagnostic reagents and analyzers.
Germany leads the European market due to its potent laboratory infrastructure and integration of digital platforms for diagnostic data management. The adoption of fully automated analyzers with connectivity to hospital information systems (HIS) and laboratory information systems (LIS) is enhancing workflow efficiency. Public-private collaborations supporting laboratory digitization and early detection of haemostatic disorders further strengthen the market landscape.
Middle East & Africa Market Insights:
The Middle East and Africa region is experiencing steady growth, supported by targeted healthcare investments in tertiary care facilities and growing collaborations with international diagnostic companies. The rise of specialized hematology and transfusion medicine centers is increasing access to advanced coagulation testing technologies. Governments are prioritizing the modernization of clinical laboratories and promoting training programs to enhance diagnostic capabilities.
In South Africa, market expansion is driven by the development of private laboratory networks and the adoption of fully automated coagulation systems within hospital settings. Partnerships with global diagnostic firms are improving access to consumables and reagents, while ongoing public health initiatives aimed at managing bleeding disorders and thrombosis are stimulating consistent demand for efficient hemostasis testing solutions.
Latin America Market Insights
Latin America is witnessing gradual growth, fueled by improving diagnostic infrastructure, increasing clinical awareness of coagulation disorders, and the inclusion of hemostasis testing within routine laboratory workflows. The growing emphasis on preventive healthcare and expansion of insurance coverage for diagnostic services are supporting broader adoption of automated analyzers and reagent systems.
Brazil leads the regional market due to strong investments in healthcare modernization and the expansion of both public and private laboratories equipped with advanced coagulation analyzers. National health programs focusing on early detection of bleeding disorders and thrombotic conditions are stimulating steady market demand, complemented by an increasing shift toward digitalized and quality-assured laboratory operations.
Product Insights
Reagents and consumables dominate the hemostasis diagnostics market with a revenue share of 65.24% due to their essential role in routine coagulation testing and continuous laboratory operations. The recurring demand for assay kits, calibrators, quality controls, and buffers ensures steady revenue generation. Advancements in reagent formulations that enhance analytical precision and stability have strengthened product reliability. Additionally, the increasing global testing volume driven by preoperative screening, anticoagulant therapy monitoring, and critical care diagnostics sustains consistent utilization of these consumables in both hospital and independent laboratory settings.
Point-of-care (POC) testing systems are anticipated to register the fastest growth of 5.22% over the forecast period, supported by their growing adoption in emergency and surgical units where rapid coagulation assessment is critical.
Test Insights
The Prothrombin Time (PT) test segment dominates the hemostasis diagnostics market with a revenue share of 33.45%, as it remains the cornerstone for assessing extrinsic and common coagulation pathways. It is routinely used to monitor patients on warfarin or other vitamin K antagonists, making it indispensable in anticoagulant therapy management. The increasing prevalence of thromboembolic disorders and liver diseases, where PT serves as a key diagnostic indicator, contributes to its extensive use. Continuous automation of PT testing and standardization of international normalized ratio (INR) reporting are also enhancing its clinical reliability and market prominence.
The D-Dimer test segment is projected to record the fastest growth of 5.52%, driven by its critical role in ruling out venous thromboembolism (VTE) and pulmonary embolism (PE). The rising global burden of cardiovascular and coagulation-related complications, combined with expanding emergency department testing volumes, is driving adoption. Technological improvements enabling high-sensitivity and rapid D-Dimer assays suitable for both laboratory and point-of-care use are further propelling the expansion. The growing incorporation of D-Dimer testing into diagnostic algorithms for COVID-19–related coagulopathies has also strengthened its demand across healthcare systems.
End User Insights
Hospitals dominate the hemostasis diagnostics market with a revenue share of 61.23% owing to their potent laboratory infrastructure, continuous patient monitoring requirements, and availability of specialized hematology and transfusion departments. The integration of automated analyzers within hospital networks enables high-volume testing, supporting critical care and surgical decision-making. Increasing incidence of coagulation disorders among hospitalized patients and the growing use of coagulation testing for preoperative and intensive care management are further enhancing the hospital segment’s market leadership.
Diagnostic laboratories are expected to exhibit the fastest growth rate of 5.32% during the forecast period, fueled by the rising demand for outsourced laboratory testing and the shift toward centralized, high-throughput testing environments. The proliferation of private and chain laboratories equipped with automated coagulation analyzers has expanded access to comprehensive testing panels. Growing adoption of digital platforms for data reporting, coupled with rising awareness of preventive diagnostics, is further boosting the utilization of coagulation testing services in diagnostic laboratory networks.
Pie Chart: Segmentation by End User in 2025 (%)
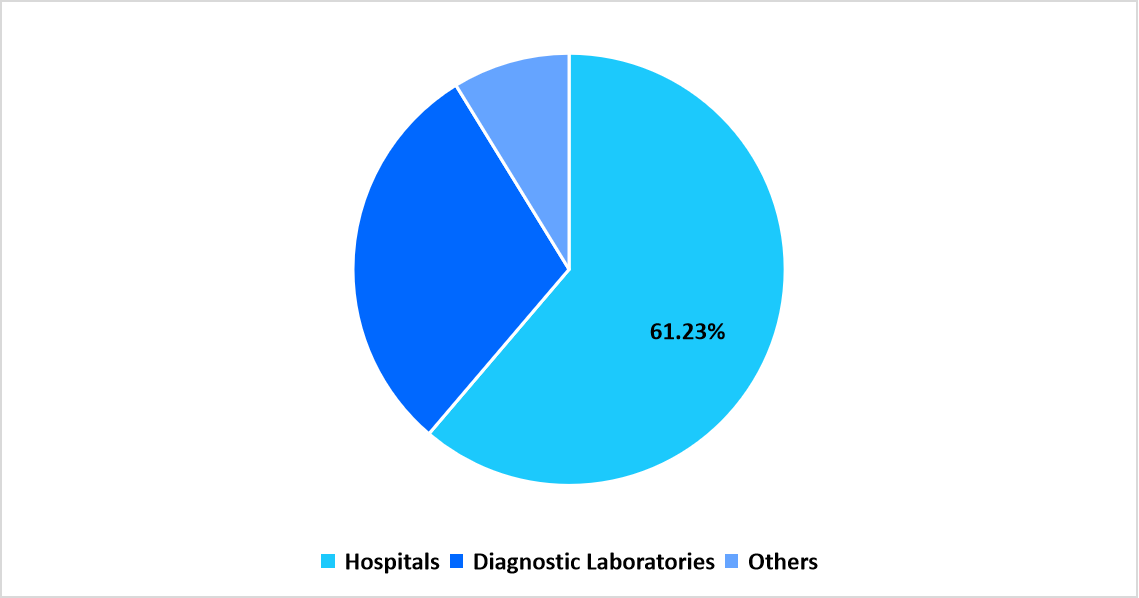
Source: Straits Research
Competitive Landscape
The global hemostasis diagnostics market is moderately competitive, characterized by the presence of established multinational corporations alongside emerging diagnostic innovators specializing in coagulation testing systems, reagents, and point-of-care solutions. Key industry participants are focusing on expanding assay portfolios, enhancing automation capabilities, and integrating advanced analytical technologies to strengthen clinical efficiency and accuracy in haemostatic testing. Strategic collaborations, technological advancements, and regulatory approvals are central to maintaining a competitive edge within the market.
Werfen: An emerging market player
Werfen is a prominent player in the hemostasis diagnostics market, recognized for its advanced coagulation testing solutions and dedicated portfolio under the Instrumentation Laboratory (IL) brand. The company focuses on delivering high-performance analyzers, reagents, and quality control systems designed for accurate and efficient coagulation assessment in both central laboratories and point-of-care settings.
In June 2024, Werfen introduced enhancements to its ACL TOP Family 50 Series analyzers, integrating advanced automation and connectivity features to streamline workflow and minimize turnaround time in clinical laboratories. These systems were compatible with Werfen’s HEMOSIL reagents, ensuring precise and consistent results across a broad range of coagulation assays. This innovation underscored Werfen’s commitment to improving laboratory efficiency, patient outcomes, and diagnostic accuracy in hemostasis testing.
List of Key and Emerging Players in Hemostasis Diagnostics Market
- Abbott
- Danaher Corporation
- Hoffmann-La Roche Ltd
- Grifols, S.A.
- Werfen
- NIHON KOHDEN CORPORATION
- HORIBA
- Bio-Rad Laboratories, Inc.
- Helena Laboratories Corporation
- Haemonetics Corporation
- CSL
- Baxter
- BD
- Siemens Healthineers AG
- Sysmex Europe SE
- Thermo Fisher Scientific Inc.
- Beckman Coulter, Inc.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Stago Group
- Others
Strategic Initiatives
- May 2025: Merit Medical acquired hemostatic device maker Biolife for USD 120 million to broaden its vascular closure and hemostasis product suite.
- January 2025: bioMérieux purchased SpinChip Diagnostics for USD 141.8 million, adding a rapid whole-blood immunoassay platform capable of 10-minute results.
- September 2024: Sysmex Corporation introduced the HISCL HIT IgG Assay Kit, designed to detect IgG antibodies in patients receiving heparin therapy. The newly launched assay kit is compatible with the company’s CN-6500 and CN-3500 analyzers, enhancing the accuracy and efficiency of heparin-induced thrombocytopenia testing.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.13 Billion |
| Market Size in 2026 | USD 2.22 Billion |
| Market Size in 2034 | USD 3.21 Billion |
| CAGR | 4.72% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Test, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Hemostasis Diagnostics Market Segments
By Product
-
Laboratory Analyzers
- Automated Systems
- Semi-automated Systems
- Manual Systems
- Point-of-Care Testing Systems
- Reagents & Consumables
By Test
- Activated Partial Thromboplastin Time
- D-Dimer Test
- Fibrinogen Test
- Prothrombin Time (PT) Test
- Other Tests
By End User
- Hospitals
- Diagnostic Laboratories
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































