Hepatic Fibrosis Market Size, Share & Trends Analysis Report By Treatment Type (Peroxisome Proliferator-Activated Receptors (Par)-Alpha Agonist, Ace Inhibitors, Hepatotropic Drug, Others), By Condition (Chronic Liver Diseases, Hepatitis C, Non-alcoholic Steatohepatitis), By End User (Hospitals, Specialty Clinics, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Hepatic Fibrosis Market Overview
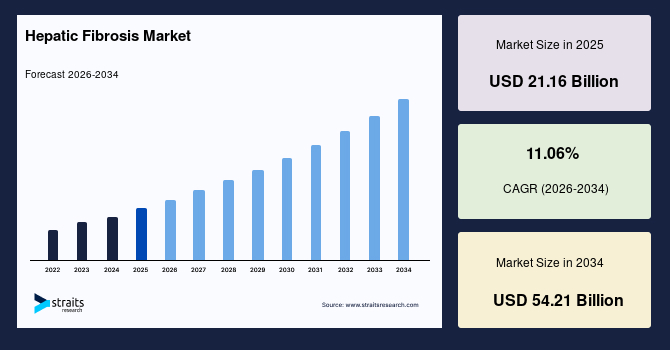
The global hepatic fibrosis market size is estimated at USD 21.16 billion in 2025 and is projected to reach USD 54.21 billion by 2034, growing at a CAGR of 11.06% during the forecast period. Remarkable growth of the market is propelled by the emergence of precision hepatology platforms that integrate metabolic signatures, genomic inputs, and fibrosis progression indicators to support personalized disease management pathways.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 40.13% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.06%.
- Based on Treatment Type, Hepatotropic Drug segment dominated the market with 42.34% share.
- Based on Condition, the Hepatitis C segment dominated the market with a 32.32% share.
- Based on End User, the Hospitals segment dominated the market with a 57.32% share.
- The U.S. dominates the global hepatic fibrosis market, valued at USD 6.86 billion in 2024 and reaching USD 7.59 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
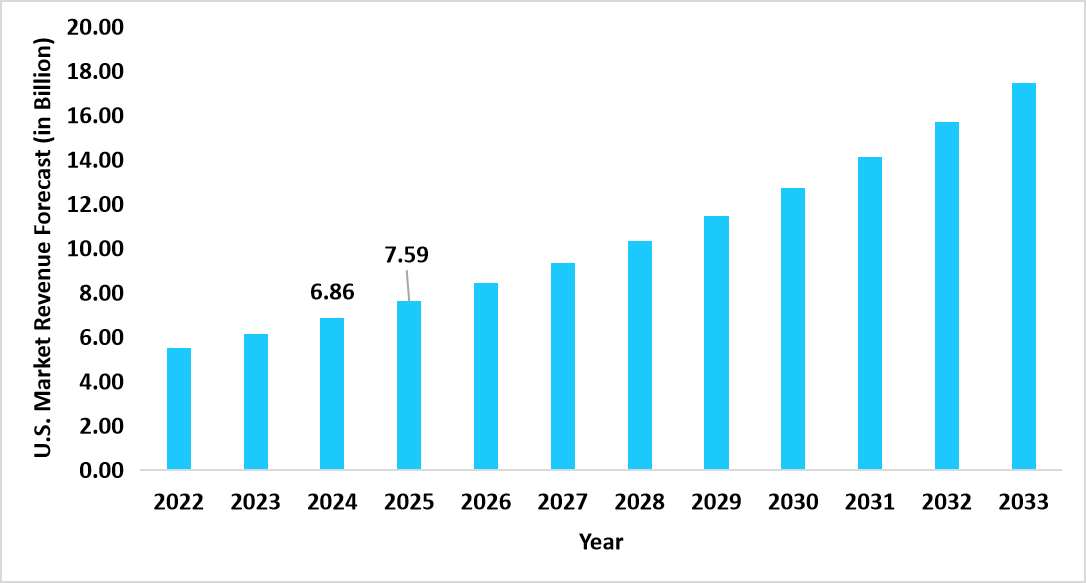
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 21.16 billion
- 2034 Projected Market Size: USD 54.21 billion
- CAGR (2025 to 2034): 11.06%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The hepatic fibrosis market encompasses the products, therapies, diagnostics, and clinical services used to assess, manage, and slow the progression of liver scarring caused by chronic conditions such as hepatitis C, non-alcoholic steatohepatitis, and other long-standing liver diseases. The market includes treatment categories such as peroxisome proliferator-activated receptor alpha agonists, ACE inhibitors, hepatotropic drugs, and related therapeutic approaches aimed at reducing inflammation and supporting liver function. It also involves a broad set of end users, including hospitals that conduct advanced staging and treatment procedures, specialty clinics that manage ongoing fibrosis monitoring, and other healthcare facilities engaged in long-term liver care. Overall, the market reflects rising clinical focus on early detection, expanded use of non-invasive diagnostic tools, and ongoing development of targeted therapies addressing progressive liver damage.
Latest Market Trends
Rising Use of Multi-Modal Biomarker Panels for Fibrosis Progression Tracking
A growing trend in the hepatic fibrosis market is the adoption of integrated biomarker panels that combine serum indicators, functional liver scores, and molecular signatures to track subtle shifts in fibrosis stages. Researchers are exploring composite panels that capture metabolic stress, stellate cell activity, and collagen turnover in a single assessment. This approach attracts attention across clinical studies seeking deeper insight into disease pathways and patient stratification.
Expansion of Computational Models for Fibrosis Risk Forecasting
The major emerging trend is the use of computational modeling platforms that evaluate lifestyle inputs, metabolic variables, and genetic predispositions to estimate future fibrosis progression. Developers are integrating longitudinal datasets with clinical markers to create predictive tools that forecast long-term liver health trajectories. These models support early identification of high-risk groups and encourage closer monitoring across treatment pathways.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 21.16 Billion |
| Estimated 2026 Value | USD 23.42 Billion |
| Projected 2034 Value | USD 54.21 Billion |
| CAGR (2026-2034) | 11.06% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Gilead Sciences, Inc. , AbbVie, Pfizer Inc., Hoffmann-La Roche Ltd, Bristol-Myers Squibb Company |
to learn more about this report Download Free Sample Report
Hepatic Fibrosis Market Driver
Growing Clinical Interest in Pathway-Targeted Antifibrotic Agents
A key driver for the market comes from rising emphasis on therapies that target cellular mechanisms linked to fibrosis formation. Pharmaceutical companies are directing resources toward agents acting on stellate cell activation, inflammatory cascades, and extracellular matrix accumulation. This expanding pipeline increases clinical activity and draws further investment into research centered on fibrosis reversal.
Market Restraint
Restricted Availability of High-Precision Fibrosis Scoring Tools in Resource-Limited Settings
A major restraint lies in uneven access to advanced fibrosis evaluation technologies in regions with constrained healthcare infrastructure. Limited distribution of elastography systems, molecular assays, and digital scoring platforms reduces the ability to conduct routine assessments. This gap slows early detection and limits enrollment in clinical studies that require consistent staging data.
Market Opportunity
Development of Personalized Therapeutic Pathways Driven by Genomic and Metabolic Profiling
A strong opportunity emerges from designing individualized treatment pathways based on genomic insights, metabolic fingerprints, and patient-specific progression markers. Tailored models can guide therapy selection, monitoring intervals, and lifestyle recommendations. This approach opens space for precision hepatology programs that enhance long-term disease management and support wider adoption of advanced fibrosis interventions.
Regional Analysis
North America holds a dominating position with a 40.13% share in the hepatic fibrosis market due to the strong availability of therapeutic programs, high adoption of advanced diagnostic pathways, and wide utilization of fibrosis assessment tools across healthcare networks. The region witnesses steady use of treatments addressing viral, metabolic, and toxin-driven liver damage. Growth is shaped by rising focus on early detection of fibrosis stages, broader use of imaging-based scoring techniques, and expanded access to specialty hepatology centers. Companies continue to progress clinical studies targeting metabolic pathways, anti-inflammatory routes, and biomarker-based monitoring.
The U.S. market expands due to a rising number of patients undergoing fibrosis staging and specialist consultations. Adoption increases as large hospital systems integrate non-invasive scoring tests into routine care. Pharmaceutical developers maintain strong activity in phase two and phase three trials targeting fibrosis regression. Reimbursement coverage for metabolic liver disease further elevates treatment uptake. Telehealth-based hepatology programs broaden access to liver assessments across suburban and rural regions.
Asia Pacific Market Insights
Asia Pacific records the fastest growth of 13.06% driven by rising prevalence of metabolic liver disorders, increased uptake of diagnostic evaluations, and expanding patient access to tertiary care facilities. Hospitals across the region are incorporating imaging-based fibrosis grading into their clinical workflows. Rising participation in screening programs for liver disease supports greater awareness of fibrosis progression across broad population groups. Digital health platforms encourage patients to complete regular follow-ups and monitor their liver profiles.
India’s market advances as rising rates of lifestyle-associated liver disorders lead to wider use of fibrosis screening. Uptake increases across tier one and tier two cities where specialized gastroenterology units are expanding. Availability of ultrasound-based elastography systems supports broader clinical use of fibrosis scoring. Domestic pharmaceutical companies are entering the segment with new trials focused on liver inflammation reduction and fibrosis stabilization. E-commerce pharmacy platforms support wider distribution of supportive therapeutics for chronic liver disorders.
Pie Chart: Regional Market Share, 2025
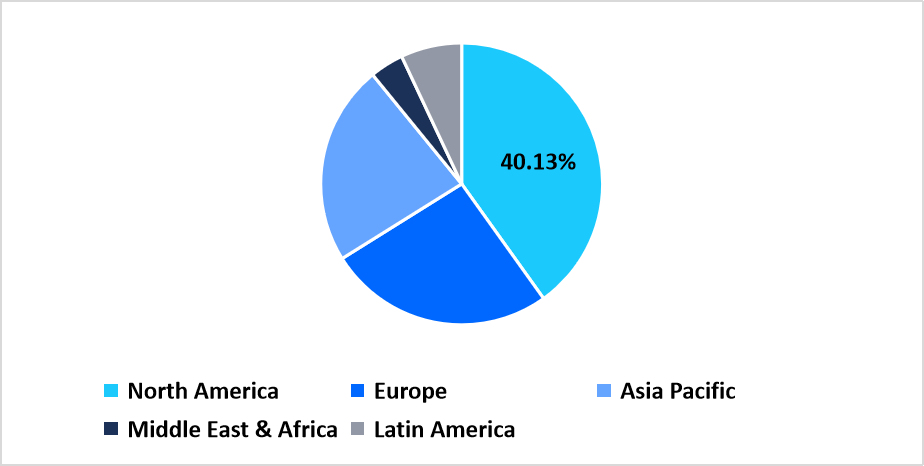
Source: Straits Research
Europe Market Insights
Europe progresses through rising uptake of diagnostic algorithms, strengthened clinical focus on early-stage liver disease management, and advancing research networks dedicated to fibrosis biology. Healthcare practitioners across the region adopt standardized fibrosis scoring protocols, improving detection across primary care and specialist units. Patients are increasingly opting for structured monitoring to track disease progression, supporting stable market expansion for therapeutics and diagnostics.
Germany’s market grows due to high utilization of elastography examinations, strong training programs for hepatology practitioners, and a strong focus on structured disease monitoring. Hospitals maintain broad access to metabolic and antiviral therapies addressing fibrosis drivers. Clinical research groups in the country continue to examine emerging mechanisms targeting hepatic stellate cell activity, drawing interest from global pharmaceutical partners. Distribution of diagnostic systems through hospital networks supports rising patient throughput.
Middle East and Africa Market Insights
The Middle East and Africa region shows forward movement as urban healthcare facilities expand hepatology services, and screening for liver disease gains wider participation. The distribution of fibrosis assessment technologies is slowly reaching larger cities, allowing healthcare providers to perform more structured evaluations. Growing awareness programs conducted by private and public organizations support increased participation in chronic liver disease check-ups.
The United Arab Emirates market advances through growing demand for liver screening programs, broadening adoption of elastography-based evaluations, and increasing availability of hepatology specialists in major hospitals. Medical tourism contributes to the uptake of fibrosis assessments as patients seek comprehensive liver evaluations. International pharmaceutical companies maintain a strong presence through distributor networks in Dubai and Abu Dhabi, improving access to systemic therapies used for fibrosis management.
Latin America Market Insights
Latin America progresses with rising utilization of liver imaging, improving access to specialty hepatology units, and growing clinical focus on metabolic liver disorders across urban regions. Hospitals and diagnostic centers are increasingly offering non-invasive fibrosis evaluations to map disease stages. Online healthcare portals broaden patient engagement and enable follow ups for chronic liver conditions.
Brazil’s market strengthens with expanding diagnostic capacity in major cities, broader public awareness of chronic liver conditions, and rising demand for non invasive fibrosis assessments. Public and private hospitals are investing in elastography and liver biomarker testing. Academic centers support research collaborations evaluating fibrosis pathways, attracting industry trials. Pharmaceutical distributors are widening access to liver disease therapeutics through both hospital and retail channels.
Treatment Type Insights
Hepatotropic drugs dominated the market with a share of 42.34 percent, driven by wide use across patients presenting with varying stages of fibrosis. Clinicians rely on these therapeutics to manage liver function and stabilize disease progression across chronic metabolic, viral, and toxin-related cases. The segment holds steady as hospitals and specialty centers continue to prescribe these medications as part of routine management pathways for broad patient groups.
Peroxisome proliferator-activated receptors alpha agonists represented the fastest growing segment with 12.45%, supported by rising clinical activity targeting metabolic liver disorders. Research advancements surrounding agents acting on PPAR pathways have elevated interest in this category, drawing attention from pharmaceutical developers working toward fibrosis regression and inflammation control. Increasing visibility of these mechanisms across clinical studies fuels continued momentum for this segment.
Condition Insights
Hepatitis C dominated the market with 32.32%, reflecting ongoing use of fibrosis staging and monitoring in populations undergoing antiviral treatment or long-term follow-up. Healthcare systems continue to track liver damage among individuals with past or present infection, which maintains high testing volumes and steady therapeutic engagement. Treatment centers observe consistent demand for fibrosis assessments among patients with chronic or resolved infection histories.
Nonalcoholic steatohepatitis recorded the fastest growth at 12.32% driven by the rising prevalence of metabolic liver disorders across global populations. Growing clinical attention toward early-stage evaluation of steatohepatitis encourages more patients to undergo fibrosis scoring and monitoring. Pharmaceutical interest in therapies for metabolic liver disease adds further traction to this category, increasing the flow of patients entering diagnostic and management pathways.
End User Insights
Hospitals dominated the market with 57.32%, as large patient volumes and access to full-service hepatology units allow hospitals to perform advanced fibrosis grading, imaging, and laboratory evaluations. Hospital settings manage complex cases that require specialist intervention, making them the primary hub for both diagnostics and ongoing treatment of fibrosis across varying etiologies.
Specialty clinics represented the fastest-growing segment with 12.01%, driven by rising demand for focused hepatology consultations and streamlined diagnostic workflows. These clinics are expanding their use of elastography, biomarker testing, and metabolic disease management programs, attracting patients seeking regular monitoring and tailored interventions. Their growing role in chronic liver disease follow-up supports steady expansion of this category.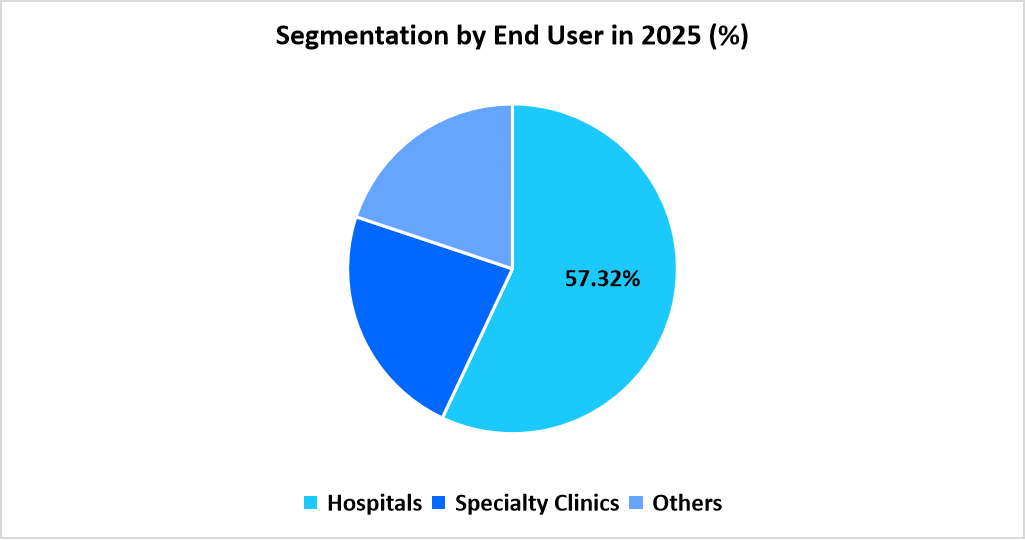
Source: Straits Research
Competitive Landscape
The hepatic fibrosis market is moderately fragmented, with a mix of large, diversified multinational pharmaceutical companies that bring broad R&D and commercialization strength, alongside smaller biotech specialists and diagnostics firms that focus on niche mechanisms, novel antifibrotic molecules, or point-of-care and genomic diagnostics.
Gilead Sciences, Inc.- An emerging market player
Gilead Sciences, Inc. is recognized for its strong portfolio in viral hepatitis and broad hepatology R&D. The company competes through late-stage assets, collaborations and acquisitions that expand its therapeutic reach into antifibrotic and metabolic liver disease programs.
List of Key and Emerging Players in Hepatic Fibrosis Market
- Gilead Sciences, Inc.
- AbbVie
- Pfizer Inc.
- Hoffmann-La Roche Ltd
- Bristol-Myers Squibb Company
- Merck & Co., Inc.
- Novartis AG
- GSK plc.
- Takeda Pharmaceutical Company Limited
- Boehringer Ingelheim International GmbH
- Galectin Therapeutics
- Intercept Pharmaceuticals, Inc.
- Can-Fite
- Enanta Pharmaceuticals, Inc.
- BioLineRx Ltd
- Others
Strategic Initiatives
- November 2025: As per EMJ’s 2024 update, semaglutide was found to have significantly reduced liver fibrosis and resolved MASH in clinical evaluations, marking a key advancement in therapeutic strategies targeting hepatic fibrosis.
- August 2024: Lumos Diagnostics announced that it had expanded its existing agreement with the Burnet Diagnostics Initiative to develop a point-of-care test designed to support liver function assessment in patients with hepatic fibrosis.
- March 2024: Gilead Sciences, Inc. completed the acquisition of CymaBay Therapeutics, which provided the company with an enhanced therapeutic portfolio for primary biliary cholangitis and strengthened its research capabilities in advancing liver disease treatments, including hepatic fibrosis.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 21.16 Billion |
| Market Size in 2026 | USD 23.42 Billion |
| Market Size in 2034 | USD 54.21 Billion |
| CAGR | 11.06% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Treatment Type, By Condition, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Hepatic Fibrosis Market Segments
By Treatment Type
- Peroxisome Proliferator-Activated Receptors (Par)-Alpha Agonist
- Ace Inhibitors
- Hepatotropic Drug
- Others
By Condition
- Chronic Liver Diseases
- Hepatitis C
- Non-alcoholic Steatohepatitis
By End User
- Hospitals
- Specialty Clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































