Hepatitis Therapeutics Market Size, Share & Trends Analysis Report By Disease Type (Hepatitis A, Hepatitis B, Hepatitis C, Others), By Drug Class (Interferons, Monoclonal Antibodies, NS5A Inhibitors, Nucleotide Analog NS5B Inhibitors, Multi-class Combinations, Other Drug Classes), By Route of Administration (Oral, Injectable), Distribution Channel (Hospital Pharmacies, Drug Stores and Retail Pharmacies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Hepatitis Therapeutics Market Overview
The global hepatitis therapeutics market size is estimated at USD 17.59 billion in 2025 and is projected to reach USD 24.90 billion in 2034, growing at a CAGR of 3.98% during the forecast period. The remarkable growth of the market is due to the rising adoption of advanced antiviral combinations that support broader treatment coverage across diverse patient groups.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 41.24% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 5.98%.
- Based on Disease Type, the Hepatitis C segment dominates the market with a revenue share of 84.52%.
- Based on Drug Class, the NS5A inhibitors segment dominates the market with a revenue share of 36.23%.
- Based on the Route of Administration, the injectable segment is anticipated to register the fastest growth of 4.44% during the forecast period.
- Based on Distribution Channel, the hospital pharmacies segment dominates the market with a revenue share of 54.25%.
- The U.S. dominates the global market, valued at USD 6.33 billion in 2024 and reaching USD 6.56 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
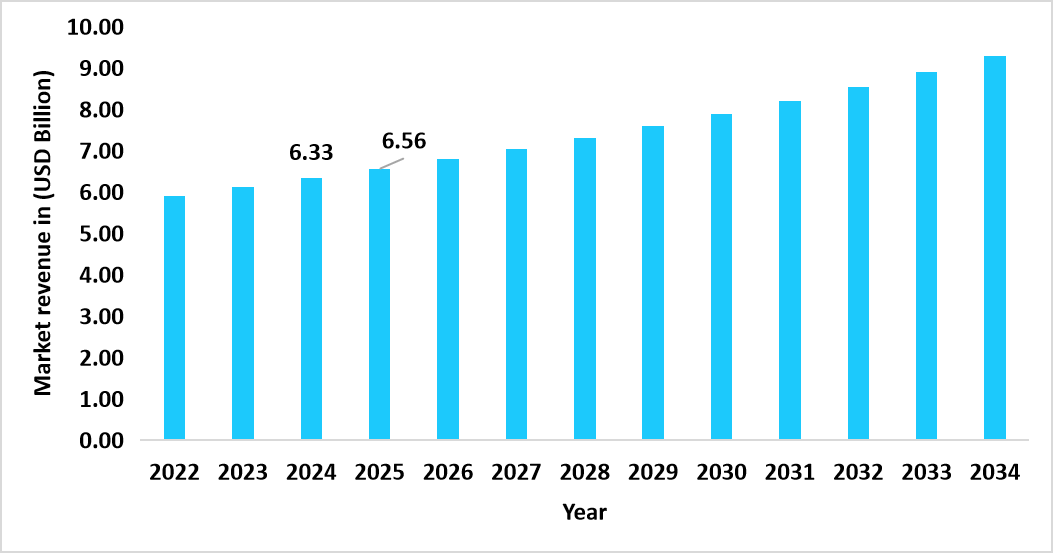
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 17.59 billion
- 2034 Projected Market Size: USD 24.90 billion
- CAGR (2025 to 2034): 3.98%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The hepatitis therapeutics market is structured across disease type, drug class, route of administration, and distribution channel, reflecting a diverse landscape shaped by evolving clinical needs and treatment patterns. By disease type, hepatitis A, hepatitis B, hepatitis C, and other forms of viral hepatitis create distinct patient groups with varying therapeutic approaches. Drug classes span interferons, monoclonal antibodies, NS5A inhibitors, nucleotide analog NS5B inhibitors, multi-class combinations, and additional therapies used across chronic and acute conditions. Routes of administration include oral regimens that support broad outpatient use and injectable options applied within supervised clinical environments. Distribution occurs through hospital pharmacies that manage complex treatment pathways, drug stores and retail pharmacies that expand community access to antiviral agents, and additional channels supplying therapies across varied healthcare settings.
Latest Market Trends
Shift from Long Duration Monotherapy to Short Course Combination Regimens
The hepatitis therapeutics landscape is moving away from extended monotherapy protocols toward short-course combinations designed to target viral replication across multiple pathways. Earlier treatment models relied on single-agent dosing that required extended periods of adherence with varied outcomes. Current approaches pursue multi-component regimens that coordinate antiviral pressure and support more rapid reduction of viral markers. This transition encourages treatment planning that aligns therapy duration with improved patient convenience and broader clinical objectives centered on durable viral suppression.
Shift from General Liver Function Monitoring to Biomarker-Driven Treatment Guidance
Clinical management is shifting from broad liver function assessments to treatment guidance shaped by biomarker panels that detect subtle changes in viral activity and immune response. Traditional monitoring centered on periodic enzyme evaluations, but emerging frameworks emphasize markers that reflect viral transcription activity, antigen expression, and host mediated control. This movement supports decision-making that adjusts therapy based on evolving biological signals rather than fixed timelines, creating a more refined structure for ongoing management of chronic hepatitis conditions.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 17.59 Billion |
| Estimated 2026 Value | USD 18.23 Billion |
| Projected 2034 Value | USD 24.90 Billion |
| CAGR (2026-2034) | 3.98% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Zydus Lifesciences Ltd., Hoffmann-La Roche Ltd., Cipla, Viatris Inc., Hetero Healthcare Limited. |
to learn more about this report Download Free Sample Report
Hepatitis Therapeutics Market Driver
Rising Adoption of Community-Based Screening Campaigns Expanding the Treated Population
Growth in community-level screening campaigns conducted through local health centers, mobile units, and outreach programs continues to expand the number of individuals diagnosed with chronic hepatitis infections. Wider identification of untreated cases introduces new groups into clinical pathways and encourages earlier discussion of therapeutic options. As detection rates rise across urban and rural zones, treatment demand increases across healthcare networks, strengthening overall market activity for antiviral regimens and supportive therapies.
Market Restraint
Fragmented Treatment Access Across Regions Limiting Consistent Therapeutic Uptake
Access to hepatitis therapeutics varies widely across healthcare systems, creating uneven patterns of treatment initiation. Regions with constrained budgets or limited specialist networks experience slower integration of advanced antiviral options, reducing the continuity of care across broader populations. These disparities contribute to delayed therapy initiation, varied adherence outcomes, and reduced capacity for coordinated national disease management strategies, restricting the overall pace of market growth.
Market Opportunity
Expansion of Cure Focused Research Platforms Enabling New Entrants in Pipeline Development
Growing engagement in cure-oriented research platforms centered on viral clearance and immune restoration is attracting new biotechnology and academic groups to the hepatitis field. This environment supports fresh exploration of therapeutic combinations that integrate molecular targeting, immune modulation, and RNA-based strategies. As these platforms evolve, they create openings for partnerships, licensing activity, and diversified clinical portfolios that strengthen long-term development prospects for companies seeking entry into the hepatitis therapeutics market.
Regional Analysis
North America maintains a strong position in the hepatitis therapeutics landscape with 41.24% share due to advanced pharmaceutical infrastructure, broad treatment accessibility, and continued expansion of antiviral development programs across academic and industry settings. Structured screening initiatives support early detection of viral hepatitis and sustain interest in treatment optimization. Coordination between government agencies, clinical networks, and research institutions encourages progress across new therapeutic approaches.
The U.S. hepatitis therapeutics market expands through rising uptake of updated antiviral regimens, steady initiation of clinical trials for HBV cure-oriented combinations, and ongoing monitoring programs across federal and state agencies. University-based research groups increase participation in early-stage trials while commercial manufacturers pursue broader distribution of approved therapies for chronic hepatitis B and C across diverse patient groups.
Asia Pacific Market Insights
Asia Pacific reflects the fastest growth of 5.98% driven by higher viral hepatitis prevalence, expanding treatment access programs, and broader national commitments to screening and disease management across China, India, Japan, South Korea, and Southeast Asia. Regional ministries advance procurement pathways for new antiviral options and strengthen community awareness strategies that support ongoing treatment adherence. Climatic variation, population density, and evolving healthcare policies create rising demand for updated therapeutic solutions.
The Chinese hepatitis therapeutics market progresses through steady enhancement of provincial screening initiatives, expansion of domestic antiviral production capacity, and wider integration of hepatitis management within urban health centers. Research institutes continue to pursue HBV-focused programs while regional authorities expand clinical participation for new therapeutic candidates.
Pie Chart: Regional Market Share, 2025

Source: Straits Research
Europe Market Insights
Europe advances its market through unified disease management strategies, structured treatment guidelines, and coordinated surveillance programs across member states. Public health agencies maintain strong collaboration with clinical research groups to evaluate therapeutic outcomes across varied population groups. Growth in migration, medical travel, and liver disease burden increases focus on optimized antiviral distribution across the region.
The UK hepatitis therapeutics market develops through broader outreach programs within primary care settings, continued updates to treatment algorithms, and expansion of specialist centers offering advanced antiviral options. Academic partnerships examine treatment responses across diverse demographics, while national guidelines encourage consistent screening within high exposure groups.
Middle East and Africa Market Insights
The Middle East and Africa region develops its market through rising urbanization, broader recognition of hepatitis related liver disease, and gradual strengthening of national treatment frameworks across Gulf and African nations. Cross-border collaborations support shared surveillance programs, while the expansion of laboratory networks enhances disease confirmation capacity. Travel to regions with high viral transmission encourages additional focus on therapeutic readiness.
The South Africa hepatitis therapeutics market evolves through targeted screening programs in public health facilities, expansion of training initiatives for healthcare workers, and increased participation in global studies assessing viral hepatitis trends. Development of diagnostic centers across major provinces improves detection accuracy and supports integration of new antiviral options within treatment pathways.
Latin America Market Insights
Latin America plays a central role in the hepatitis therapeutics landscape due to elevated liver disease burden, long-standing public health engagement, and continued commitment to treatment access programs. Regional authorities promote structured introduction of updated antiviral regimens while collaborative work across Brazil, Mexico, Argentina, and Colombia strengthens surveillance networks. Urban healthcare expansion provides broader capacity for treatment delivery.
The Argentina hepatitis therapeutics market advances through revisions in national viral hepatitis management plans, integration of treatment counselling in community clinics, and expansion of government supported research projects focused on HBV and HCV disease patterns. Growth in domestic pharmaceutical and bioscience activities enhances participation in clinical evaluations and prepares the region for broader adoption of new therapeutic options.
Disease Type Insights
Hepatitis C dominates with 84.52%, supported by broad utilization of direct-acting antiviral combinations that continue to shape treatment decisions across global healthcare systems. The availability of simplified oral regimens with high cure potential encourages wide adoption among diagnosed populations, while structured screening programs across various regions increase the number of patients entering therapy. Clinical outcomes associated with shorter treatment durations further reinforce the continued preference for Hepatitis C regimens across outpatient and specialty care settings.
Hepatitis B records the fastest growth at 4.91%, driven by expanding research interest in functional cure approaches and steady advancement of pipeline candidates centered on immune targeting and viral suppression strategies. Broader national initiatives promoting screening and linkage to care support earlier initiation of therapy, bringing more individuals into treatment pathways. Increasing recognition of untreated chronic Hepatitis B as a long-term driver of liver disease encourages healthcare systems to adopt more aggressive management frameworks, contributing to the upward trajectory of this segment.
Drug Class Insights
NS5A inhibitors dominate with 36.23%, reflecting their established integration within leading direct-acting antiviral regimens for Hepatitis C. These agents remain central components of once daily oral therapies used across a broad spectrum of patient profiles, including treatment-naive individuals and those with complex clinical histories. Their inclusion in multiple fixed-dose combinations strengthens continued clinical reliance, while real-world evidence across various populations supports sustained utilization across global markets.
Monoclonal antibodies record the fastest growth at 4.32%, supported by ongoing development programs exploring immune pathway modulation for chronic Hepatitis B and Hepatitis D. New candidates under evaluation aim to alter viral persistence through targeted mechanisms, drawing continued investment from biotechnology and pharmaceutical developers. Growing interest in combination strategies that include monoclonal antibodies within multi-agent regimens expands the future potential of this segment, making it a rapidly advancing category within the hepatitis treatment landscape.
Route of Administration Insights
The oral segment dominates in 2025, supported by widespread acceptance of simplified dosing formats that align with outpatient care models and reduce treatment burden for individuals. Oral antivirals remain the foundation of Hepatitis C management and continue to be integrated into evolving Hepatitis B treatment strategies. Convenience of administration, predictable adherence patterns, and ease of distribution through both hospital and retail channels reinforce the sustained prominence of oral therapies across various regions.
The injectable segment records the fastest growth with 4.44%, driven by rising utilization of biologics and emerging immunotherapies that require administration under clinical supervision. These treatments are gaining attention due to their potential roles within Hepatitis B and Hepatitis D combination regimens. The growing availability of specialized infusion centers and increased clinician familiarity with injectable antiviral modalities contribute to the expanding presence of this segment.
Distribution Channel Insights
Hospital pharmacies dominate with 54.25%, supported by their central role in managing complex hepatitis cases, coordinating specialist oversight, and ensuring access to advanced antiviral regimens. Hospitals maintain structured pathways for patient evaluation, follow-up, and adherence monitoring, resulting in concentrated dispensing of therapies within these institutional settings. Integration of hepatology units and infectious disease departments further strengthens the dominance of hospital pharmacies across the hepatitis treatment ecosystem.
Drug stores and retail pharmacies record the fastest growth at 4.65%, driven by expanding distribution of oral hepatitis medications through community-level outlets that improve convenience for patients. Wider participation of retail pharmacies in chronic disease management programs increases accessibility for individuals requiring routine antiviral therapy. Growth in decentralized healthcare models, along with the broader geographic reach of retail networks, supports the rising prominence of this channel across varied populations.
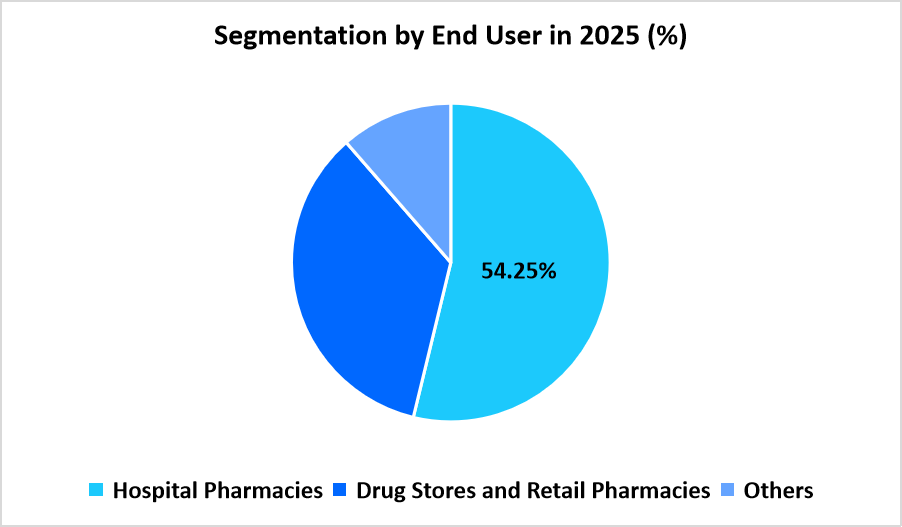
Source: Straits Research
Competitive Landscape
The hepatitis therapeutics market includes established pharmaceutical companies, regional drug manufacturers, and research focused biotechnology firms expanding treatment pipelines across antiviral classes for hepatitis B, C, and D.
Arbutus Biopharma: An emerging market player
Arbutus Biopharma concentrated on hepatitis B cure oriented research supported by its advancing core inhibitor programs and RNA targeting therapeutic strategies. The company expanded preclinical and clinical evaluations aimed at reducing viral replication, lowering surface antigen levels, and shaping long term immune control in chronic HBV. Arbutus also continued collaborations and portfolio refinements to strengthen combination regimens designed to move toward sustained virologic responses in diverse patient groups.
List of Key and Emerging Players in Hepatitis Therapeutics Market
- Zydus Lifesciences Ltd.
- Hoffmann-La Roche Ltd.
- Cipla
- Viatris Inc.
- Hetero Healthcare Limited.
- Arbutus Biopharma
- Assembly Biosciences, Inc.
- Aligos Therapeutics
- Dynavax Technologies.
- Enanta Pharmaceuticals, Inc.
- Gilead Sciences, Inc.
- Abbvie, Inc.
- Bristol-Myers Squibb Company
- GSK
- Merck & Co., Inc.
- Others
Strategic Initiatives
- August 2024: Bristol Myers Squibb announced that the U.S. FDA accepted the supplemental Biologics License Application (sBLA) for Opdivo plus Yervoy as a potential first-line treatment for adults with unresectable hepatocellular carcinoma.
- May 2025: AbbVie obtained FDA approval for an 8-week MAVYRET regimen to treat acute hepatitis C, reporting a 96% cure rate.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 17.59 Billion |
| Market Size in 2026 | USD 18.23 Billion |
| Market Size in 2034 | USD 24.90 Billion |
| CAGR | 3.98% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Disease Type, By Drug Class, By Route of Administration, Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Hepatitis Therapeutics Market Segments
By Disease Type
- Hepatitis A
- Hepatitis B
- Hepatitis C
- Others
By Drug Class
- Interferons
- Monoclonal Antibodies
- NS5A Inhibitors
- Nucleotide Analog NS5B Inhibitors
- Multi-class Combinations
- Other Drug Classes
By Route of Administration
- Oral
- Injectable
Distribution Channel
- Hospital Pharmacies
- Drug Stores and Retail Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































