In Vitro Cancer Diagnostics Market Size, Share & Trends Analysis Report By Offering (2026-2034) (Product, Services), By Technology (2026-2034) (Clinical Chemistry, Immunochemistry/Immunoassays, Hematology, Coagulation, Hemostasis, Microbiology, Molecular Diagnostics, Others), By End User (2026-2034) (Hospitals & Clinics, Diagnostic Laboratories, Homecare, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
In Vitro Cancer Diagnostics Market Overview
The global in vitro cancer diagnostics market size is valued at USD 20.41 billion in 2025 and is estimated to reach USD 32.96 billion by 2034, growing at a CAGR of 5.51% during the forecast period. The consistent growth of the market is supported by the adoption of biomarker-based companion diagnostics, enabling personalized cancer treatment.
Key Market Trends & Insights
- North America dominated the global market, accounting for a 40.07% share in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 6.87%.
- Based on offering, the product segment dominated the market in 2025.
- By application, the immunochemistry/immunoassays segment dominated the market with 28.61% in 2025.
- Based on end user, the hospitals & clinics segment dominated the market in 2025.
- The U.S. dominates the market, valued at USD 7.02 billion in 2024 and reaching USD 7.38 billion in 2025.
Table: U.S. In Vitro Cancer Diagnostics Market Size (USD Million)
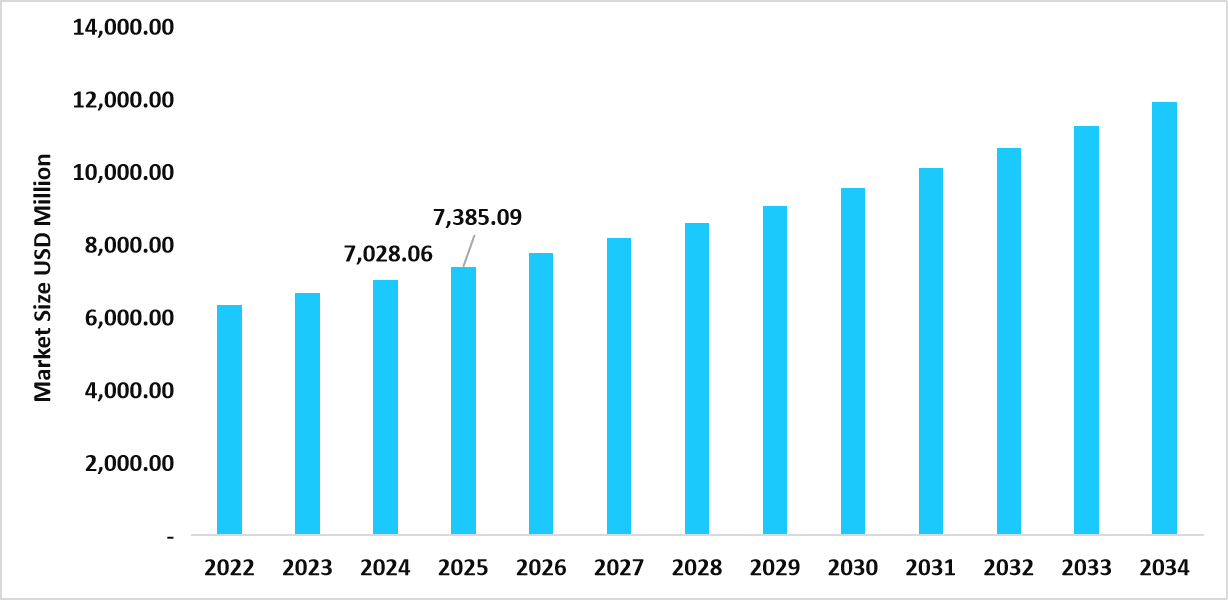
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 20.41 billion
- 2034 Projected Market Size: USD 32.96 billion
- CAGR (2026-2034): 5.51%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The global in vitro cancer diagnostics market encompasses a diverse range of offerings, including instruments, reagents & kits, and associated diagnostic services, enabling accurate detection, monitoring, and management of various cancers. The market leverages multiple technologies, such as clinical chemistry, immunoassays, hematology, coagulation, hemostasis, microbiology, and molecular diagnostics, to deliver precise and timely results. IVD cancer diagnostics are utilized across hospitals, diagnostic laboratories, home care settings, and other healthcare facilities.
Latest Market Trends
Shift Towards Liquid Biopsy And Molecular Diagnostics Over Conventional Tissue Biopsy
The growing adoption of liquid biopsy and advanced molecular diagnostics is a key trend in the global in vitro cancer diagnostics market. Companies such as Roche and Guardant Health are expanding their portfolio of circulating tumor DNA (ctDNA) tests for non invasive cancer detection and monitoring.
This shift from traditional tissue biopsy to minimally invasive diagnostics is enhancing early detection and patient compliance, which, in turn, drives market growth.
Integration Of AI and Digital Technologies In Cancer Diagnostics
The increasing integration of artificial intelligence and digital tools in in vitro cancer diagnostics is a major trend shaping the market. For example, Freenome leverages multiomics technology combined with AI to develop intelligent screening platforms that enhance early cancer detection. This shift from conventional interpretation to AI augmented workflows is improving diagnostic accuracy, efficiency, and patient outcomes, supporting wider adoption of advanced cancer diagnostics in hospitals and laboratories globally.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 20.41 Billion |
| Estimated 2026 Value | USD 21.46 Billion |
| Projected 2034 Value | USD 32.96 Billion |
| CAGR (2026-2034) | 5.51% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Alcon Inc., Johnson & Johnson Vision Care, Inc., Bausch + Lomb Incorporated, Carl Zeiss Meditec AG, HOYA Corporation |
to learn more about this report Download Free Sample Report
Market Drivers
Expansion of government-funded cancer screening programmes
The expansion of large-scale, government-funded cancer screening initiatives is emerging as a major driver of the global in vitro cancer diagnostics market. For instance, screening participation for early detection tools increased by approximately 40% over the past five years, driven by public awareness campaigns and programmatic roll‑outs. This transition from symptomatic testing to structured population-based screening is widening the diagnostic testing base, enabling earlier detection, higher diagnostic volumes, and broader market uptake of advanced IVD cancer diagnostics.
Market Restraints
Regulatory Complexities and Lengthy Approval Processes
A key restraint in the IVD cancer diagnostics market is the complexity of regulatory approvals across different regions. Diagnostic assays, especially molecular and next-generation sequencing tests, must comply with stringent regulations from authorities such as the FDA, EMA, and other regional bodies. These lengthy approval timelines delay product launches, increase development costs, and limit rapid adoption of innovative diagnostic technologies globally.
Market Opportunities
Expansion of Personalized Medicine
The shift toward personalized medicine in oncology is creating growth opportunities for the in vitro cancer diagnostics market. Personalized medicine relies on understanding a patient’s specific tumor biology to guide targeted therapy decisions, which require advanced diagnostic tests such as molecular profiling, genomic sequencing, and biomarker analysis. For example, 75% of oncology drugs approved between 2020 and 2024 now require companion diagnostic tests. This increases demand for accurate IVD cancer diagnostics, creating new growth opportunities.
Regional Analysis
North America dominated the in vitro cancer diagnostics market in 2025, accounting for 40.07% market share. This growth is augmented by the strong presence of advanced genomic testing infrastructure, supported by extensive R&D investments and early technology adoption. The region’s robust laboratory networks and precision oncology platforms provide rapid implementation of innovative cancer diagnostics, reinforcing its leadership in the IVD landscape.
U.S. market growth is driven by the expansion of decentralized molecular testing in community hospitals and regional labs. This shift brings advanced cancer diagnostics closer to patients, improving accessibility, reducing turnaround times, and enhancing early detection across healthcare networks.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 6.87% from 2026 to 2034, owing to the rising establishment of localized IVD manufacturing hubs in countries like China, Singapore, and South Korea. These hubs reduce import dependency, lower production costs, and enable faster regional distribution of cancer diagnostic products, strengthening the region’s self-sufficiency and market competitiveness.
India's market growth is supported by the emergence of indigenous molecular diagnostic startups developing advanced cancer testing kits. Companies such as Molbio Diagnostics and MedGenome are advancing affordable genomic and PCR-based solutions tailored for Indian healthcare, fostering innovation and accessibility in cancer diagnostics.
Regional Market share (%) in 2025
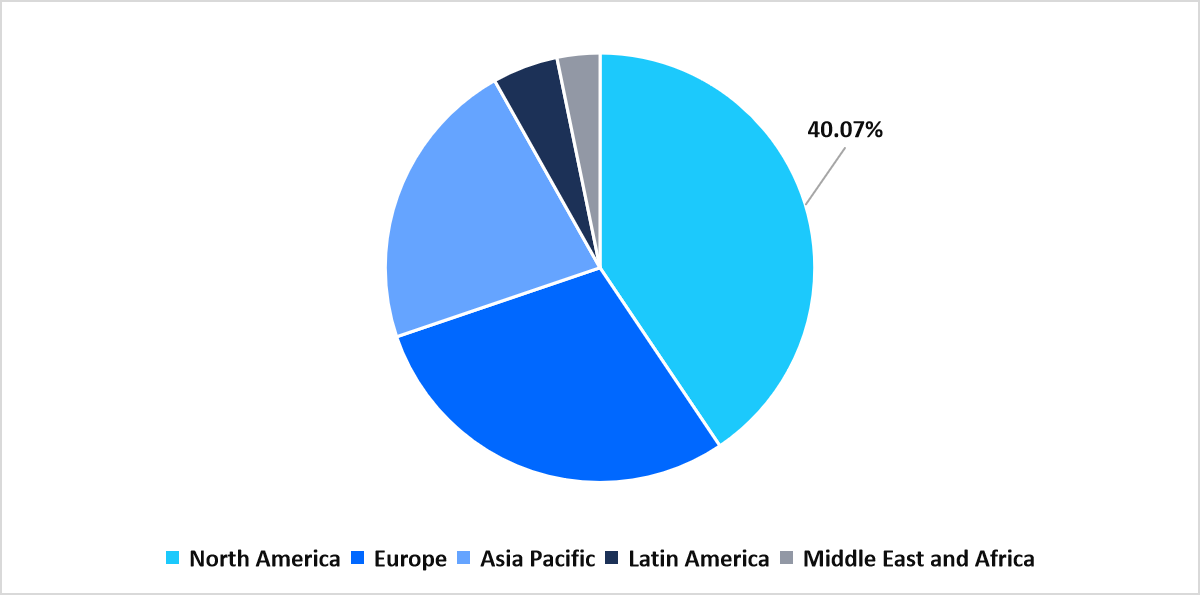
Source: Straits Analysis
Europe Market Insights
Europe's in vitro cancer diagnostics market growth is supported by the implementation of the In Vitro Diagnostic Regulation (IVDR), which harmonizes diagnostic standards across EU member states. These regulatory factors enhance product quality, promote intercity commercialization, and accelerate the adoption of advanced cancer diagnostic assays through greater safety and compliance transparency across the region.
In Germany, the market is experiencing notable growth, driven by a structured reimbursement framework that covers molecular and biomarker-based cancer tests under statutory health insurance. This financial accessibility encourages hospitals and laboratories to adopt advanced diagnostic technologies, boosting utilization rates and driving continuous innovation within the country’s in vitro cancer diagnostics sector.
Latin America Market Insights
Latin America’s in vitro cancer diagnostics market is driven by the rapid expansion of regional diagnostic reference laboratories equipped with advanced molecular testing technologies. Countries such as Brazil, Mexico, and Chile are strengthening centralized lab networks to enhance cancer detection accuracy, streamline test processing, and improve accessibility to advanced in vitro diagnostic services across diverse populations.
The growth of Brazil's market is driven by the rapid expansion of regional diagnostic reference laboratories equipped with advanced molecular testing technologies. Brazil is also strengthening centralized lab networks to enhance cancer detection accuracy, streamline test processing, and improve accessibility to advanced in vitro diagnostic services across diverse populations.
Middle East and Africa Market Insights
The Middle East and Africa market is growing due to the establishment of specialized oncology diagnostic centers in Gulf countries such as the UAE and Saudi Arabia. These centers integrate advanced molecular testing and automation, improving cancer detection accuracy, strengthening regional healthcare infrastructure, and fostering technology transfer across emerging African markets.
South Africa’s market growth is driven by the adoption of mobile and decentralized cancer diagnostic testing models to reach remote and underserved communities. Supported by public health agencies and NGOs, these initiatives enhance early detection, expand access to molecular testing, and strengthen the country’s cancer care ecosystem.
Offering Insights
The product segment dominated the market in 2025. This growth is driven by the adoption of high-throughput, fully automated instruments in healthcare settings. The QIAGEN QIAcuity Dx digital PCR system enables efficient, all-in-one cancer testing in clinical laboratories. Thus, growing adoption of advanced instruments supports segmental market growth.
The services segment is projected to register the fastest CAGR of 7.03% during the forecast period, owing to the increased adoption of outsourced diagnostic analysis services, where specialized labs provide advanced genomic and biomarker test interpretation for hospitals and smaller labs.
Technology Insights
The immunochemistry/immunoassays segment dominated the market in 2025, accounting for 28.61% revenue share. This dominance is attributed to the rising use of multiplex immunoassays that allow simultaneous measurement of cancer biomarkers in a single test, improving diagnostic efficiency, reducing sample volume, and supporting cancer profiling.
The molecular diagnostics segment is estimated to register the fastest CAGR of 6.14% during the forecast period. This growth is stimulated by the expanding availability of compact, sample platforms that allow rapid genetic mutation detection in oncology. For example, Genomtec S.A. extended its ID platform into oncology diagnostics with a novel isothermal amplification method for genetic variant detection.
By Technology Market Share (%), 2025
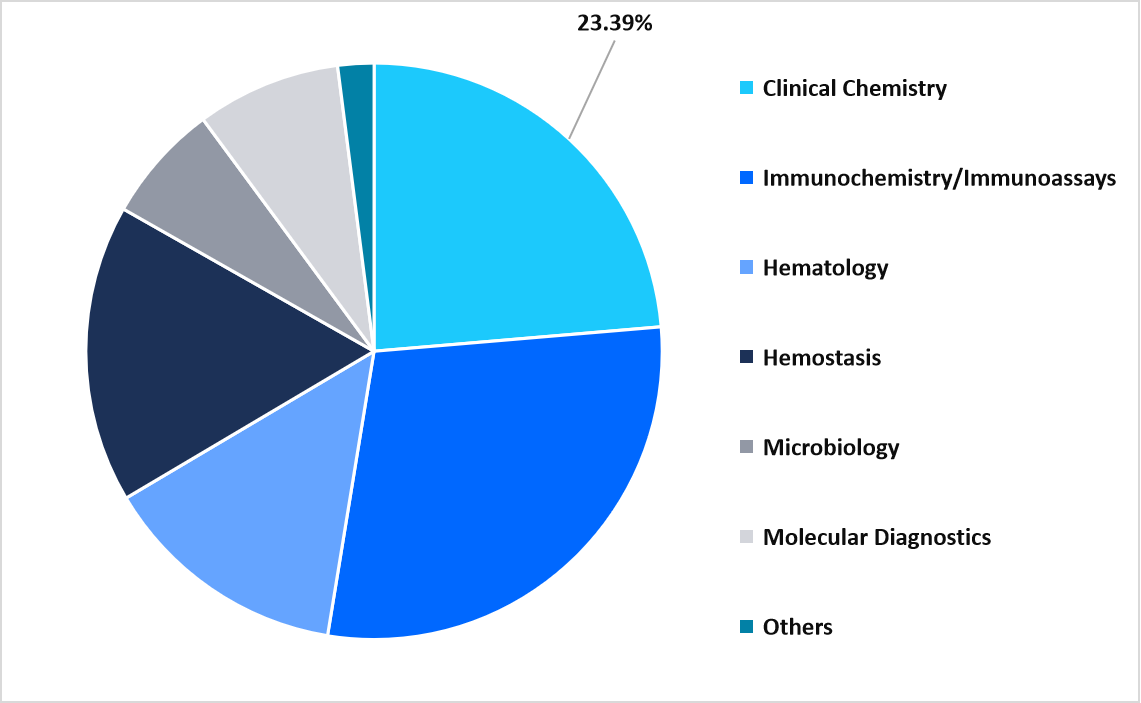
Source: Straits Analysis
End User Insights
The hospitals & clinics segment dominated the market with the highest revenue share in 2025, as hospitals and clinics are increasingly integrating advanced IVD cancer diagnostics directly into inpatient care pathways, allowing seamless coordination of testing and treatment. This streamlined workflow improves turnaround time and drives higher adoption of IVD services in these healthcare settings, thereby supporting segmental growth.
The diagnostic laboratories segment is projected to register the fastest CAGR of 6.37% from 2026-2034, due to the adoption of high-throughput centralized testing workflows, which allow laboratories to process large volumes of samples efficiently, reduce errors, improve turnaround times, and deliver faster diagnostic results.
Competitive Landscape
The global in vitro cancer diagnostics market is moderately consolidated, with a few large multinationals commanding substantial market share and a growing spectrum of emerging firms. Companies such as F. Hoffmann‑La Roche Ltd., Abbott Laboratories, Thermo Fisher Scientific Inc., Siemens Healthineers, and others dominate across assay platforms, molecular diagnostics and immunoassays. At the same time, specialized firms focusing on liquid biopsy, multi‑omics profiling, and AI‑driven diagnostics are challenging incumbents via product differentiation and strategic partnerships.
OneCell Diagnostics: An emerging market player
OneCell Diagnostics, a biotech startup, is emerging in the market with its development of a proprietary liquid biopsy platform that combines single-cell multi-omics and AI-driven analytics.
- In November 2024, OneCell Diagnostics raised USD 16 million in a Series A funding round to advance its OncoIndx Ikon platform for clinical validation.
Through its innovative diagnostic technology and successful funding, OneCell Diagnostics is positioning itself as an emerging player in the global market.
List of Key and Emerging Players in In Vitro Cancer Diagnostics Market
- Alcon Inc.
- Johnson & Johnson Vision Care, Inc.
- Bausch + Lomb Incorporated
- Carl Zeiss Meditec AG
- HOYA Corporation
- Rayner Group
- Eyekon Medical
- Lenstec, Inc.
- STAAR SURGICAL
- HumanOptics Holding AG
- Ophtec
- USIOL Inc.
- Innexus
- Oculentis GmbH
- Physiol S.A.
- Cooper Companies, Inc.
- Glaukos Corporation
- EssilorLuxottica S.A.
- Santen Pharmaceutical Co., Ltd.
- Others
Strategic Initiatives
- May 2025: Illumina Inc. expanded its clinical oncology portfolio of tumor profiling and in vitro diagnostic (IVD) solutions.
- June 2025: QIAGEN partnered with GENCURIX to develop oncology assays for use on the QIAcuityDx platform, a digital PCR system.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 20.41 Billion |
| Market Size in 2026 | USD 21.46 Billion |
| Market Size in 2034 | USD 32.96 Billion |
| CAGR | 5.51% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Offering (2026-2034), By Technology (2026-2034), By End User (2026-2034) |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
In Vitro Cancer Diagnostics Market Segments
By Offering (2026-2034)
-
Product
- Instruments
- Reagents & Kits
- Services
By Technology (2026-2034)
- Clinical Chemistry
- Immunochemistry/Immunoassays
- Hematology
- Coagulation
- Hemostasis
- Microbiology
- Molecular Diagnostics
- Others
By End User (2026-2034)
- Hospitals & Clinics
- Diagnostic Laboratories
- Homecare
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































