Isothermal Nucleic Acid Amplification Technology Market Size, Share & Trends Analysis Report By Product (Instruments, Reagent), By Technology (Transcription Mediated Amplification (TMA), Loop-mediated isothermal amplification (LAMP), Strand Displacement Amplification (SDA), Helicase-dependent Amplification (HDA), Nucleic Acid Sequence-based Amplification (NASBA), Nicking Enzyme Amplification Reaction (NEAR), Single Primer Isothermal Amplification (SPIA), Others), By Application (Blood screening, Infectious disease diagnostics, Cancer, Others), By End-use (Hospitals, Central and reference labs, Other) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Isothermal Nucleic Acid Amplification Technology Market Overview
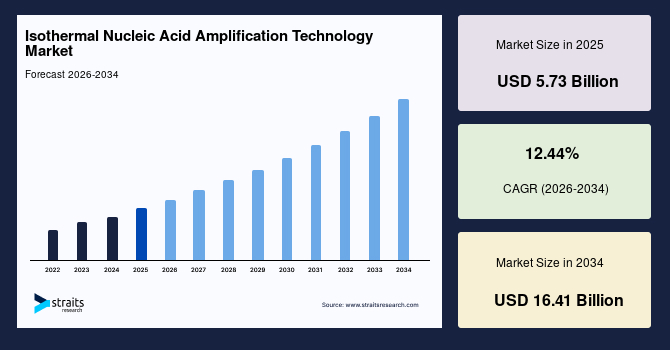
The global isothermal nucleic acid amplification technology market size is valued at USD 5.73 billion in 2025 and is estimated to reach USD 16.41 billion by 2034, growing at a CAGR of 12.44% during the forecast period. The consistent market growth is supported by the increasing adoption of rapid, point-of-care molecular diagnostics that enable faster pathogen detection without the need for complex thermal cycling equipment.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 45.84% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 14.44%.
- Based on Product, the instruments segment is anticipated to register the fastest CAGR of 13.33% during the forecast period.
- Based on Technology, the loop-mediated isothermal amplification (LAMP) segment dominated the market with a revenue share of 18.12%.
- Based on Application, the infectious disease diagnostics segment dominated the market with a revenue share of 36.71%.
- Based on End-Use, the hospital segment dominated the market with a revenue share of 41.32%.
- The U.S. dominates the isothermal nucleic acid amplification technology market, valued at USD 2.12 billion in 2024 and reaching USD 2.38 billion in 2025.
Table: U.S. Isothermal Nucleic Acid Amplification Technology Market Size (USD Billion)
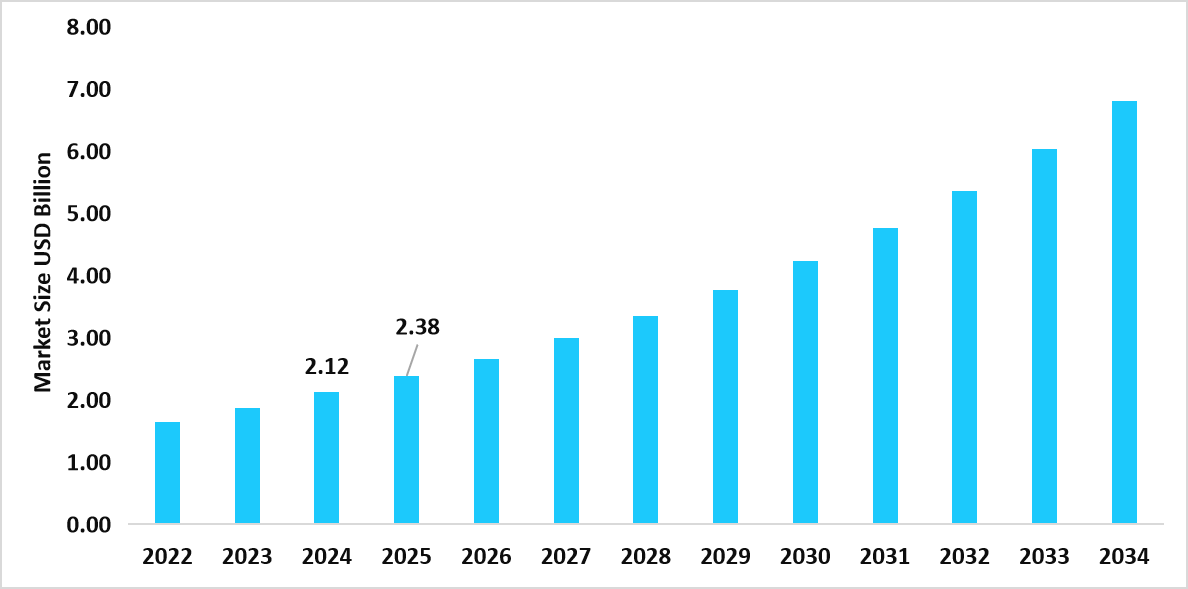
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 5.73 billion
- 2034 Projected Market Size: USD 16.41 billion
- CAGR (2026-2034): 12.44%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The isothermal nucleic acid amplification technology market comprises diagnostic tools and solutions designed to amplify nucleic acids at a constant temperature, eliminating the need for complex thermal cycling systems used in conventional PCR methods. This market includes instruments and reagents that support a range of isothermal techniques such as transcription-mediated amplification, loop-mediated isothermal amplification, strand displacement amplification, helicase-dependent amplification, nucleic acid sequence-based amplification, nicking enzyme amplification reaction, single primer isothermal amplification, and related methods.
These technologies are widely applied in blood screening, infectious disease diagnostics, oncology testing, and other molecular analysis applications due to their rapid turnaround time, operational simplicity, and suitability for decentralized testing environments. End users primarily include hospitals, central and reference laboratories, and other diagnostic settings, where isothermal amplification technologies are increasingly integrated to support timely clinical decision making and large-scale screening programs.
Latest Market Trends
Shift From Centralized Thermal Cycling PCR Workflows To Decentralized Isothermal Amplification Platforms
Laboratories and diagnostic providers are moving from equipment-intensive PCR systems toward isothermal amplification formats that operate at constant temperatures and support near-patient testing. This shift reflects a growing preference for simplified molecular workflows that can be deployed outside large laboratory infrastructures. Isothermal platforms allow faster assay initiation, reduced power dependence, and easier integration into mobile and resource-limited testing environments. As diagnostic networks expand into peripheral healthcare settings, constant temperature amplification supports wider test accessibility while maintaining molecular-level sensitivity.
Shift From Multi-Step Amplification Protocols To Integrated Assay Cartridges and Closed Systems
There is an increasing transition from open bench amplification procedures to integrated cartridges that combine amplification chemistry, detection reagents, and reaction control within a single format. These systems reduce manual handling, lower contamination risk, and standardize reaction conditions across test sites. Integrated designs are gaining adoption in infectious disease screening and blood safety testing, where consistency across high sample volumes is critical. The shift supports smoother workflow execution and aligns with the growing use of compact diagnostic instruments.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 5.73 Billion |
| Estimated 2026 Value | USD 6.42 Billion |
| Projected 2034 Value | USD 16.41 Billion |
| CAGR (2026-2034) | 12.44% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Ustar Biotechnologies (Hangzhou) Ltd., Hologic, Inc., Abbott, Thermo Fisher Scientific Inc. , QIAGEN |
to learn more about this report Download Free Sample Report
Market Driver
Rising Demand For Rapid Molecular Diagnostics With A Short Turnaround Time
The market is driven by growing demand for molecular tests that deliver results within minutes to hours rather than days. Isothermal amplification enables faster signal generation without lengthy heating cycles, making it well-suited for urgent clinical decision making. This demand is particularly strong in infectious disease management, blood screening programs, and outbreak response settings where time-sensitive results influence treatment and containment strategies.
Market Restraint
Limited Assay Multiplexing Capability Compared To Conventional PCR Methods
A key restraint is the relative complexity of developing highly multiplexed isothermal assays. Managing primer interactions and reaction specificity becomes more challenging as target numbers increase. This limitation can restrict broader adoption in applications requiring simultaneous detection of multiple biomarkers, particularly in oncology and syndromic testing panels.
Market Opportunity
Expansion Of Point-Of-Care Molecular Testing In Emerging And Remote Healthcare Settings
A major opportunity lies in the expansion of point-of-care molecular diagnostics across emerging regions and remote care environments. Isothermal amplification technologies are well-suited for these settings due to minimal instrumentation requirements and simplified operation. As healthcare systems invest in decentralized diagnostic models, adoption of portable isothermal platforms for infectious disease detection, blood screening, and community-level testing is expected to increase, supporting long-term market expansion.
Regional Analysis
North America represents a leading region for isothermal nucleic acid amplification technologies with a market share of 45.84%, supported by early adoption of rapid molecular testing platforms across clinical and public health laboratories. The region demonstrates strong utilization of constant temperature amplification systems within hospital networks, blood banks, and outpatient diagnostic centers. Integration of isothermal assays into routine molecular workflows supports fast sample processing and streamlined testing operations. Ongoing collaboration between diagnostic manufacturers and healthcare providers further reinforces regional market presence.
In the U.S., expansion is supported by increasing use of isothermal amplification assays within emergency care settings, donor screening programs, and decentralized testing sites. Healthcare institutions deploy compact amplification platforms to support rapid clinical decision-making, strengthening the country’s leadership in adoption.
Asia Pacific Market Insights
Asia Pacific registers the fastest growth at 14.44%, driven by the rising establishment of decentralized diagnostic facilities and the expansion of molecular testing capacity across urban and semi-urban regions. Laboratories increasingly adopt isothermal amplification to address high patient volumes and support outbreak monitoring initiatives. Growth of domestic diagnostic manufacturing and regional testing networks accelerates market expansion.
In China, development is supported by the large-scale deployment of rapid molecular assays across hospital laboratories and regional testing centers. Adoption of isothermal platforms for infectious disease surveillance and routine diagnostics contributes to sustained market growth.
Regional Market share (%) in 2025
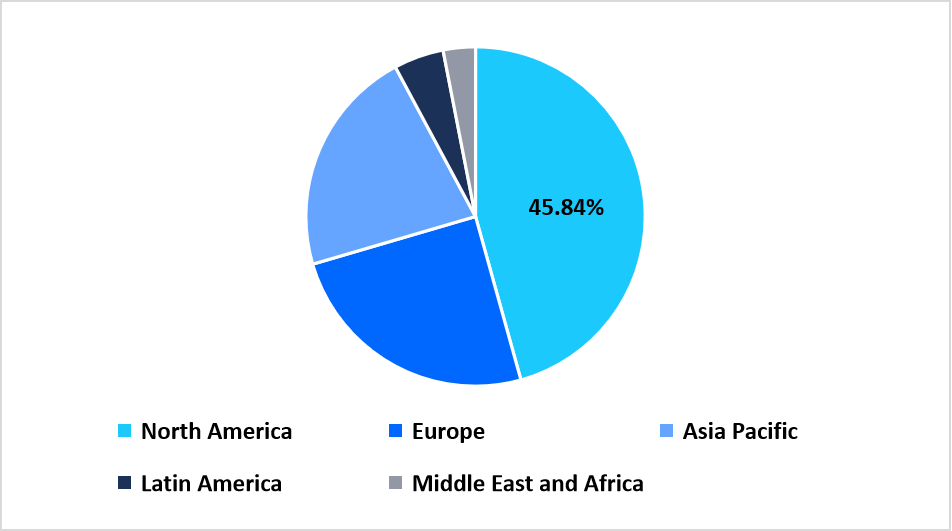
Source: Straits Research
Europe Market Insights
Europe maintains steady adoption through integration of isothermal amplification technologies into structured healthcare systems and national screening initiatives. Clinical laboratories utilize these platforms to support fast turnaround testing and standardized molecular workflows. Regional emphasis on harmonized diagnostic practices supports continued uptake.
In Germany, growth is driven by the adoption of isothermal assays within clinical microbiology laboratories and blood screening facilities. Use of rapid amplification platforms in routine diagnostics and public health testing strengthens market stability.
Latin America Market Insights
Latin America experiences gradual expansion as healthcare providers broaden access to molecular diagnostics in public hospitals and regional laboratories. Isothermal amplification systems gain traction for routine infectious disease testing and blood safety programs due to simplified operational requirements.
In Brazil, growth is reinforced by increased incorporation of rapid molecular platforms within hospital laboratories and public health institutions. Expansion of diagnostic coverage supports broader regional adoption.
Middle East and Africa Market Insights
The Middle East and Africa region advances through progressive strengthening of molecular diagnostic infrastructure across healthcare and research facilities. Adoption of isothermal amplification supports faster testing in settings with limited laboratory infrastructure. Training initiatives and capacity-building programs contribute to wider technology use.
In the United Arab Emirates, market momentum is supported by the deployment of rapid molecular testing platforms across hospitals and diagnostic centers. Integration of isothermal amplification into routine clinical workflows enhances the country’s regional presence.
Product Insights
Reagents dominated the product segment, reflecting their recurring consumption across routine diagnostic testing and large-scale screening programs. High usage frequency in isothermal assays for infectious disease detection and blood screening sustains consistent demand for amplification reagents across laboratories.
Instruments are anticipated to register the fastest growth at 13.33%, supported by increasing deployment of compact amplification platforms designed for decentralized testing environments. Adoption of portable and benchtop systems across hospitals and peripheral laboratories contributes to the accelerated growth of this segment.
Technology Insights
Loop-mediated isothermal amplification dominated the technology segment with a share of 18.12%, driven by its broad assay compatibility, rapid amplification capability, and suitability for point-of-care diagnostics. Its use in infectious disease testing and field-deployable applications supports sustained leadership.
Transcription-mediated amplification is expected to witness the fastest growth at 13.68%, supported by expanding use in high-sensitivity RNA-based assays, particularly in blood screening and viral load monitoring workflows.
Application Insights
Infectious disease diagnostics led the application segment with 36.71%, driven by extensive adoption of isothermal amplification in pathogen detection, outbreak surveillance, and rapid clinical testing programs. Continuous expansion of molecular testing volumes strengthens this segment’s dominance.
Blood screening is projected to grow at the fastest rate of 13.78%, supported by rising emphasis on transfusion safety and integration of rapid nucleic acid testing in donor screening protocols.
End Use Insights
Hospitals dominated the end-use segment with a share of 41.32%, supported by increasing utilization of rapid molecular assays within emergency care, inpatient diagnostics, and routine screening services.
Central and reference laboratories are anticipated to record the fastest growth at 13.88%, driven by expansion of large-scale testing contracts, centralized screening programs, and adoption of high-throughput isothermal platforms.
End Use Market share (%) in 2025
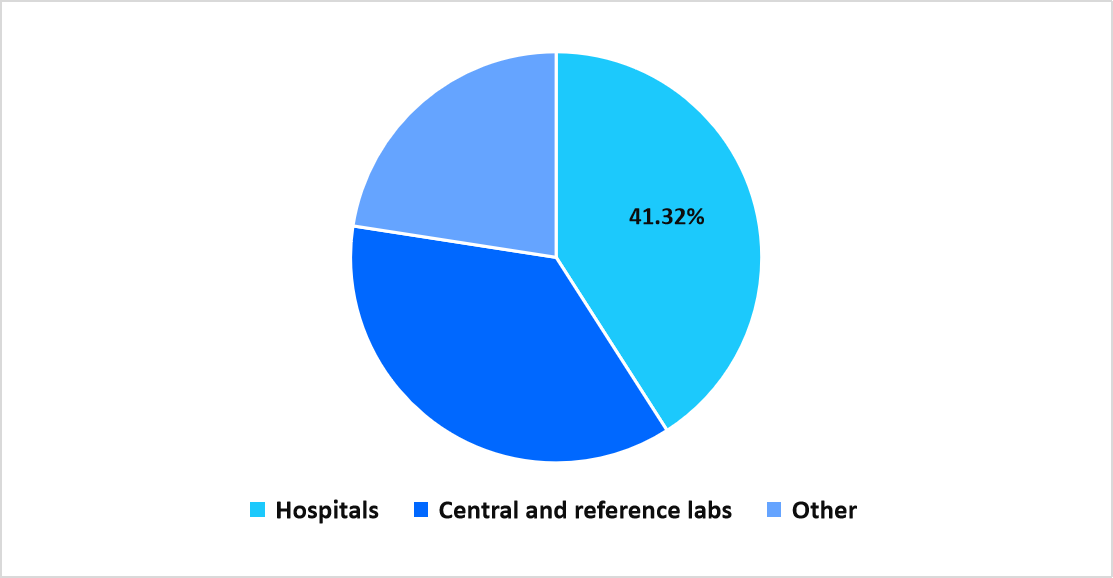
Source: Straits Research
Competitive Landscape
The global isothermal nucleic acid amplification technology market is moderately fragmented, with diagnostic assay developers, instrument manufacturers, and reagent suppliers holding established positions across clinical and laboratory testing environments. Market participants strengthen their presence through the expansion of assay menus, refinement of amplification chemistries, and development of compact platforms suited for decentralized molecular testing. Companies emphasize compatibility with rapid diagnostic workflows, regulatory clearances for clinical use, and partnerships with hospitals, blood banks, and reference laboratories. Continued focus on assay specificity, turnaround time reduction, and scalability across testing volumes supports competitive differentiation within the market.
Hologic Inc.: An Emerging Market Player
Hologic Inc. holds a strong position in the isothermal nucleic acid amplification technology market through its transcription-mediated amplification-based diagnostic platforms. The company focuses on high-sensitivity molecular assays designed for blood screening and infectious disease diagnostics. Its systems are widely adopted across centralized laboratories due to standardized workflows and consistent assay performance. Hologic continues to expand its molecular diagnostics portfolio by enhancing assay coverage and strengthening adoption across transfusion medicine and clinical testing environments.
List of Key and Emerging Players in Isothermal Nucleic Acid Amplification Technology Market
- Ustar Biotechnologies (Hangzhou) Ltd.
- Hologic, Inc.
- Abbott
- Thermo Fisher Scientific Inc.
- QIAGEN
- Meridian Bioscience
- Eiken Chemical Co., Ltd.
- GENEYE
- CANON MEDICAL SYSTEMS CORPORATION
- Sysmex Europe SE
- Enzo Biochem Inc.
- New England Biolabs
- TwistDx Limited
- Others
Strategic Initiatives
-
November 2024: The United States Centers for Disease Control and Prevention entered into a new agreement with industry partners to support the development of avian influenza diagnostic tests using isothermal nucleic acid amplification approaches. The agreement aimed to expand rapid testing capacity for avian flu detection beyond centralized public health laboratories and strengthen preparedness for emerging influenza threats.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 5.73 Billion |
| Market Size in 2026 | USD 6.42 Billion |
| Market Size in 2034 | USD 16.41 Billion |
| CAGR | 12.44% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Technology, By Application, By End-use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Isothermal Nucleic Acid Amplification Technology Market Segments
By Product
- Instruments
- Reagent
By Technology
- Transcription Mediated Amplification (TMA)
- Loop-mediated isothermal amplification (LAMP)
- Strand Displacement Amplification (SDA)
- Helicase-dependent Amplification (HDA)
- Nucleic Acid Sequence-based Amplification (NASBA)
- Nicking Enzyme Amplification Reaction (NEAR)
- Single Primer Isothermal Amplification (SPIA)
- Others
By Application
- Blood screening
- Infectious disease diagnostics
- Cancer
- Others
By End-use
- Hospitals
- Central and reference labs
- Other
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































