Life Sciences Quality Management Software Market Size, Share & Trends Analysis Report By Application (Data Management, Training Management, Supplier Management, Regulatory and Compliance Management, Corrective Action and Preventive Action (CAPA) Management, Audit Management, Change Management, Non-conformances Management, Inspection Management, Risk Management, Others), By Deployment Mode (Cloud & Web-based, On-premises), By End Use (Pharmaceutical Firms, Biotech Firms, CROs/CDMOs) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Life Sciences Quality Management Software Market Overview
The global life sciences quality management software market size is estimated at USD 3.87 billion in 2025 and is projected to reach USD 11.40 billion by 2034, growing at a CAGR of 12.78% during the forecast period. Sustained growth of the market is propelled by the rising adoption of digital quality systems across regulated manufacturing, research, and compliance operations.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 42.78%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 14.78%.
- Based on the Application, the data management segment dominated the market with a revenue share of 20.34%.
- Based on Deployment Mode, the on-premises segment is anticipated to register the fastest CAGR of 13.42%.
- Based on End Use, the pharmaceutical firms segment dominated the market with a revenue share of 59.23%
- The U.S. dominates the global life sciences quality management software market, valued at USD 1.33 billion in 2024 and reaching USD 1.50 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
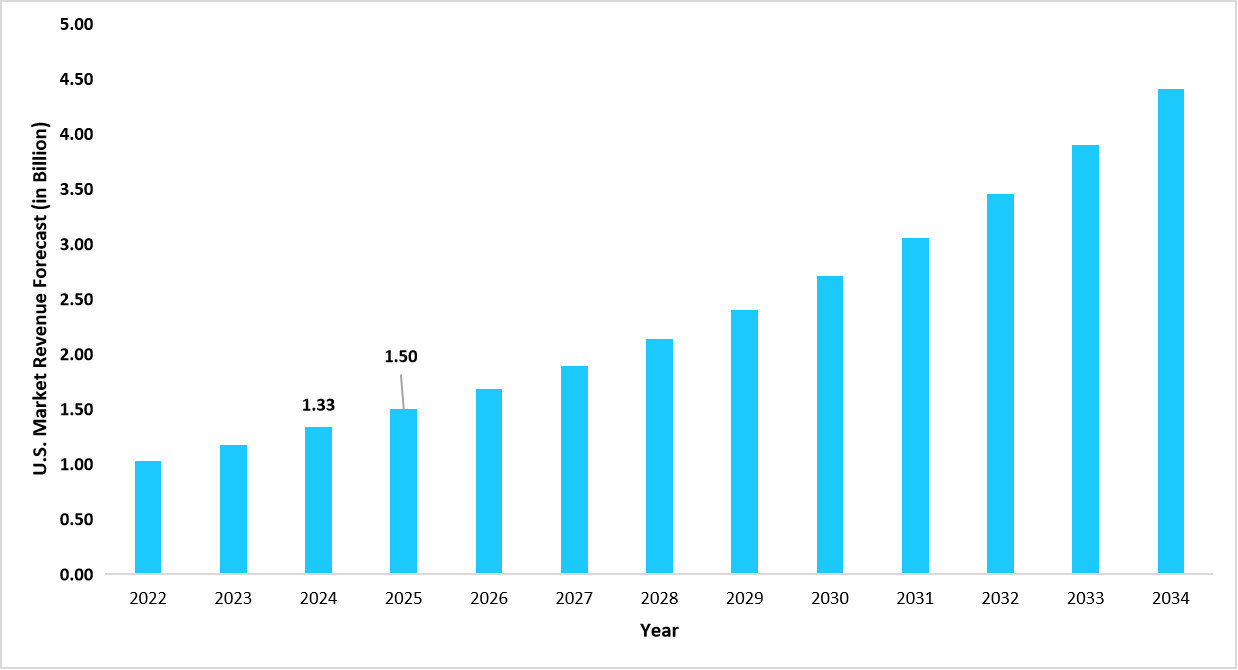
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 3.87 billion
- 2034 Projected Market Size: USD 11.40 billion
- CAGR (2025 to 2034): 12.78%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The Life Sciences Quality Management Software Market encompasses digital platforms designed to manage, monitor, and streamline quality processes across regulated pharmaceutical, biotechnology, and contract research or manufacturing environments. These software systems support a wide range of applications, including data management, training oversight, supplier evaluation, regulatory and compliance activities, corrective and preventive action tracking, audit execution, change-control workflows, non-conformance handling, inspection coordination, risk assessment, and other quality-related functions. Solutions are deployed through cloud and web-based models or on-premises infrastructures, depending on organizational requirements and governance frameworks. End users include pharmaceutical firms seeking structured quality documentation, biotech companies managing complex production cycles, and CROs/CDMOs that must maintain consistent compliance across multi-client operations.
Latest Market Trends
Rising Use of Predictive Quality Analytics for Early Deviation Detection
A niche trend in the Life Sciences Quality Management Software market is the growing use of predictive quality analytics that evaluate historical quality records to forecast potential deviations before they occur. Firms are incorporating analytical dashboards into routine production reviews, allowing earlier adjustments to process parameters. This shift strengthens proactive quality oversight and supports smoother decision-making during critical manufacturing cycles.
Integration of Digital Training Tracking with Real-Time Production Readiness Checks
The major emerging trend is the linkage of digital training modules with shop-floor readiness assessments. Systems are being configured to verify employee qualification status before task assignments, creating structured workflows that prevent skill-related errors. As facilities aim to maintain uninterrupted operations, these integrated checks are becoming part of routine quality-assurance planning.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 3.87 Billion |
| Estimated 2026 Value | USD 4.35 Billion |
| Projected 2034 Value | USD 11.40 Billion |
| CAGR (2026-2034) | 12.78% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | QT9 Software, Honeywell International Inc., Hexagon AB, Intergraph Corporation, AssurX, Inc., Veeva Systems |
to learn more about this report Download Free Sample Report
Market Driver
Growing Alignment of Supplier-Quality Programs with Centralized Digital Platforms
A key driver is the expansion of supplier-quality programs into centralized software ecosystems that gather audit results, certifications, and corrective records across global vendor networks. Life sciences companies are adopting unified supplier-quality environments to gain clearer visibility into raw-material and component sources, which strengthens compliance across outsourced manufacturing chains.
Market Restraint
Slow Transition from Paper-Based Archives in Legacy Facilities
A restraint arises from long-standing reliance on paper archives in older facilities, which slows migration to fully digital quality ecosystems. Large volumes of historical batch documents, validation reports, and audit files require extensive conversion efforts, delaying system configuration and influencing adoption timelines in legacy manufacturing units.
Market Opportunity
Expansion of Remote Qualification and Digital Workforce Competency Programs
A growing opportunity is the rise of remote qualification programs that allow employees to complete competency assessments and certification renewals through digital platforms without on-site sessions. Software vendors are introducing structured evaluation modules and remote-access reviews, expanding adoption across facilities with distributed teams and supporting long-term workforce readiness.
Regional Analysis
North America maintained a leading position with a 42.78% share driven by the wide adoption of cloud-based Life Sciences Quality Management Software across pharmaceutical, biotechnology, and medical-device companies. The region advanced due to strong regulatory oversight, broad acceptance of digital validation tools, and extensive integration of quality-management platforms with manufacturing and clinical-quality workflows. Large vendors expanded enterprise deployments across multi-site operations, strengthening digital quality coordination throughout the region.
The U.S. expanded as organizations increased the use of electronic documentation modules and electronic batch-record systems in FDA-regulated environments. Federal authorization granted to platforms such as MasterControl Quality Excellence Gov strengthened adoption within public-sector health agencies, raising the country’s share in digital-quality transformation.
Asia Pacific Market Insights
Asia Pacific emerged as the fastest-expanding region with a CAGR of 14.78% as pharmaceutical and biopharma manufacturers accelerated the installation of Life Sciences Quality Management Software to support larger production capacities. Contract manufacturing organizations adopted digital-quality platforms to manage complex operations, lifting software licensing activity across the region. Local firms transitioned from manual documentation to cloud-deployed quality-management systems to improve audit preparedness and cross-site coordination.
India advanced due to a national digital-health infrastructure that encouraged the adoption of electronic quality-management platforms within pharmaceutical facilities. Expanded use of data-integrity modules across formulation and bulk-drug plants strengthened the country’s contribution to regional software growth.
Pie Chart: Regional Market Share, 2025
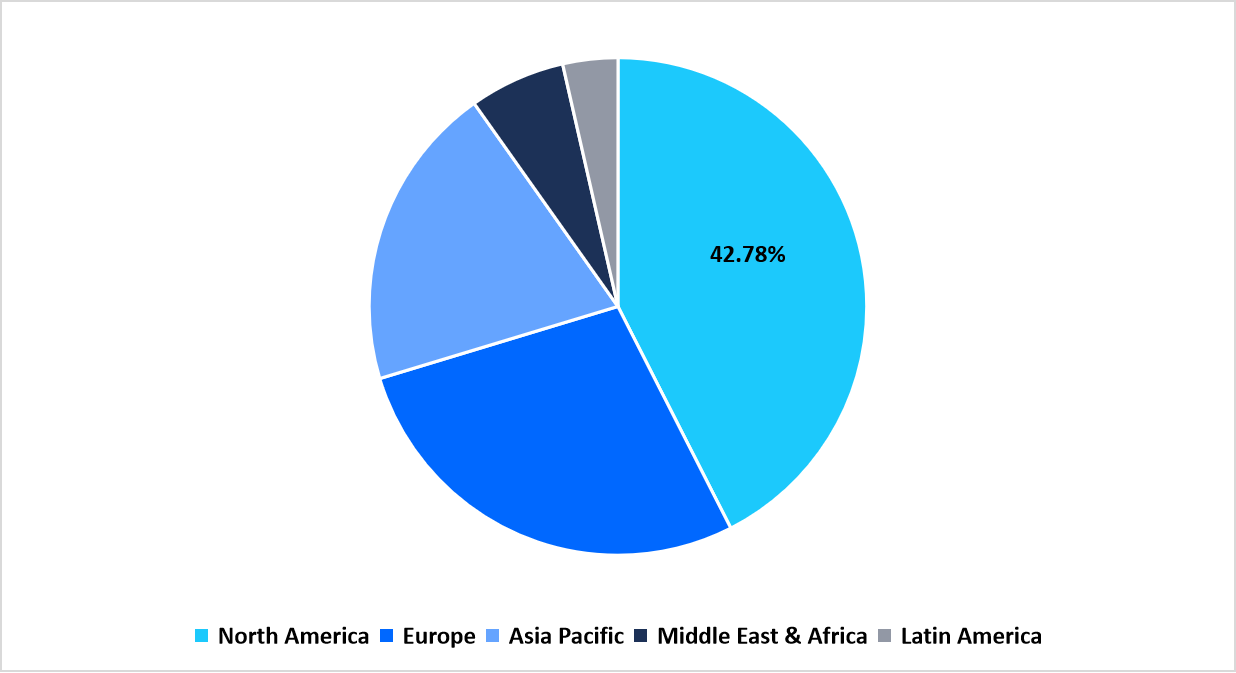
Source: Straits Research
Europe Market Insights
Europe experienced steady growth supported by harmonized quality standards across EU member states that encouraged broader installation of Life Sciences Quality Management Software. The region progressed as manufacturers upgraded older platforms to meet evolving EMA and MDR expectations for digital documentation. Expanded digitization within clinical-trial operations further boosted investment in integrated quality-management and study-management environments.
Germany strengthened its position as a major pharmaceutical and med-tech organization expanded quality-management software across research, manufacturing, and supplier-audit functions. Programs involving regulators and industry associations promoted higher alignment to electronic quality documentation, which supported rising adoption of enterprise-wide quality-management platforms.
Middle East and Africa Market Insights
Middle East and Africa recorded rising adoption as public and private life sciences organizations expanded digital-quality programs to strengthen audit-readiness and align with global regulatory expectations. Growth of pharmaceutical formulation units and biologics-focused facilities across Gulf nations increased demand for both cloud-based and on-premise Life Sciences Quality Management Software.
Saudi Arabia advanced through national digital-transformation initiatives that encouraged adoption of electronic quality documentation across manufacturing sites. Expansion of certification and compliance programs increased interest in automated workflows for deviations, training, and change-control.
Latin America Market Insights
Latin America witnesses broader adoption of Life Sciences Quality Management Software due to expanding pharmaceutical production capacity and closer collaboration with international regulatory bodies. Regional manufacturers invested in electronic documentation tools and CAPA-management systems to support export-focused operations. Partnerships with global vendors increased the availability of configurable cloud-based quality-management solutions.
Brazil progressed as state-supported digital-compliance programs encouraged the adoption of centralized quality-management software in pharmaceutical and biologics facilities. Enforced electronic audit requirements increased the installation of structured documentation, training, and supplier-quality modules across public and private production sites.
Application Insights
The Data Management segment dominated the market in 2025 with 20.34%, supported by strong adoption of centralized platforms that enabled coordinated documentation workflows across regulated production and research environments. Broader use of structured data handling enhanced traceability across quality events and sustained its leading position.
The Regulatory and Compliance Management segment recorded the fastest growth at 13.22%, driven by rising emphasis on audit-ready documentation and continuous alignment with evolving regulatory expectations. Adoption increased as organizations prioritized accurate tracking of compliance activities across manufacturing and supply-chain units.
Deployment Mode Insights
The Cloud & Web-based segment dominated the market in 2025 as life sciences companies expanded the use of remotely accessible quality-management platforms to support cross-functional operations. Wider acceptance of subscription-based models contributed to higher deployment across global sites.
The On-premises segment exhibited the fastest growth at 13.42%, driven by facilities that maintained controlled server environments and preferred local data architecture for quality documentation processes. Adoption grew in organizations with dedicated IT infrastructures overseeing system configuration and validation activities.
End Use Insights
The Pharmaceutical Firms segment dominated the market in 2025 with 59.23%, supported by extensive integration of quality-management software across formulation units, packaging lines, and analytical laboratories. High documentation volumes across daily operations contributed to strong system usage.
The Biotech Firms segment grows at the fastest pace of 13.67%, propelled by expanding biologics and advanced-therapy development pipelines that require structured quality oversight. Increased activity across emerging biomanufacturing clusters accelerated the adoption of digital quality-management platforms.
Segmentation by End Use in 2025 (%)
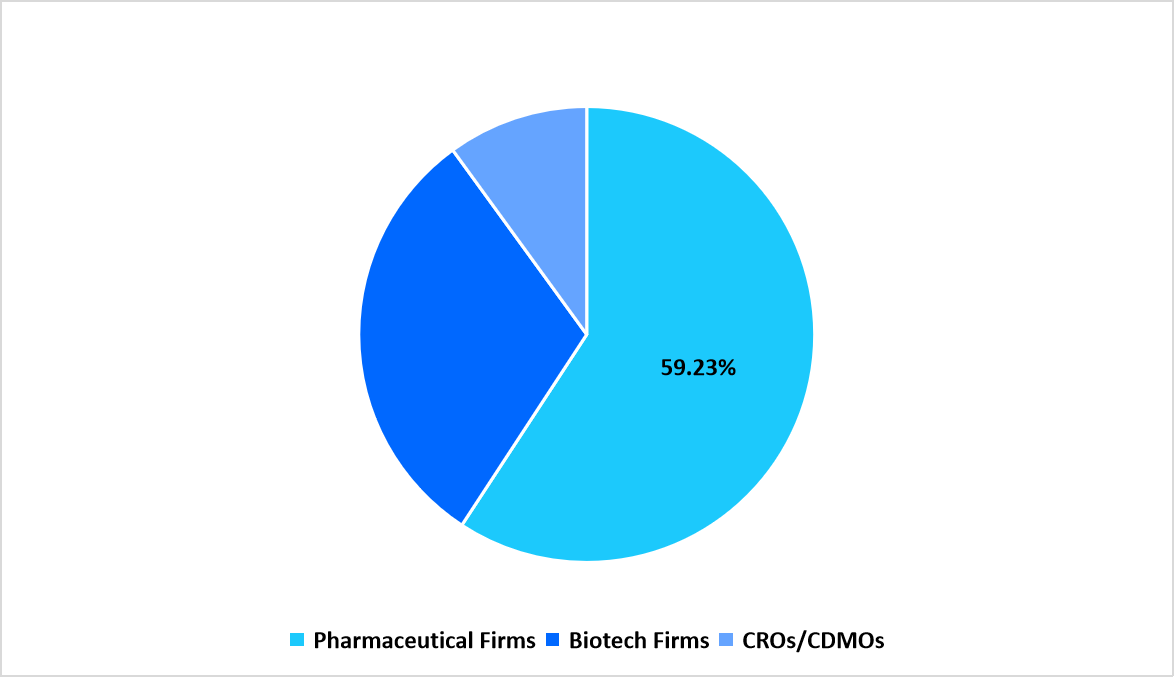
Source: Straits Research
Competitive Landscape
The global life sciences quality management software market is moderately fragmented, with activity spread across established enterprise software vendors, cloud-based QMS providers, compliance-focused technology firms, and specialized life sciences software companies that together shape a broad digital quality ecosystem. Companies compete through product enhancements, platform scalability, regulatory-aligned features, strategic alliances, and expansion into new geographic regions, as well as through continuous upgrades tailored to pharmaceutical, biotechnology, and medical-device workflows.
Veeva Systems: An Emerging Market Player
- Veeva Systems gained a strong role in the market with its Vault QMS, which integrates quality, regulatory, and content-management functions within a unified cloud platform. The company expanded its footprint across pharmaceutical and biotechnology firms by emphasizing cross-department connectivity, reducing documentation cycles, and streamlining audit-readiness across global operations.
List of Key and Emerging Players in Life Sciences Quality Management Software Market
- QT9 Software
- Honeywell International Inc.
- Hexagon AB, Intergraph Corporation
- AssurX, Inc.
- Veeva Systems
- Scilife N.V.
- Greenlight Guru.
- Qualityze
- GMPPro
- Dot Compliance Ltd.
- InstantGMP
- QMS for Life Sciences
- MasterControl Solutions, Inc.
- IQVIA
- Others
Strategic Initiatives
- May 2025: MasterControl Quality Excellence Gov (Qx Gov) received FedRAMP Moderate authorization, making it the first QMS to meet that federal cybersecurity standard.
- August 2024: Honeywell launched Honeywell Quality Management Review (HQMR), a digital application for life sciences that streamlined and automated quality management review processes.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 3.87 Billion |
| Market Size in 2026 | USD 4.35 Billion |
| Market Size in 2034 | USD 11.40 Billion |
| CAGR | 12.78% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Application, By Deployment Mode, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Life Sciences Quality Management Software Market Segments
By Application
- Data Management
- Training Management
- Supplier Management
- Regulatory and Compliance Management
- Corrective Action and Preventive Action (CAPA) Management
- Audit Management
- Change Management
- Non-conformances Management
- Inspection Management
- Risk Management
- Others
By Deployment Mode
- Cloud & Web-based
- On-premises
By End Use
- Pharmaceutical Firms
- Biotech Firms
- CROs/CDMOs
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































