Live Biotherapeutic Products And Microbiome CDMO Market Size, Share & Trends Analysis Report By Component (Solution, Services), By Type (Bacterial LBPs, Viral LBPs, Fungal LBPs, Others), By Application (Gastrointestinal Disorders, Oncology, Neurological Disorders, Infectious Diseases, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Live Biotherapeutic Products and Microbiome Cdmo Market Size
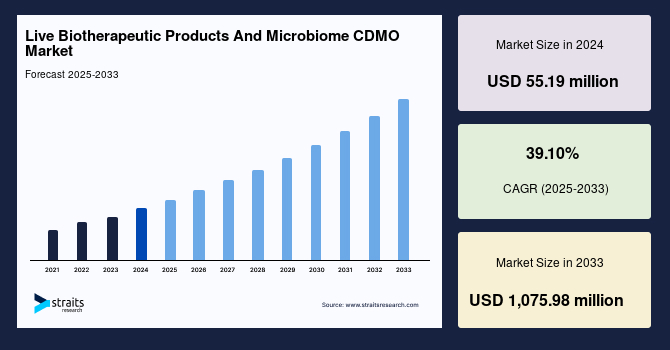
The global live biotherapeutic products and microbiome CDMO market size was valued at USD 55.19 million in 2024 and is projected to grow from USD 76.77 million in 2025 to USD 1,075.98 million in 2033, exhibiting a CAGR of 39.10% during the forecast period (2025–2033).
The global live biotherapeutic products and microbiome CDMO market is experiencing rapid growth, driven by increasing awareness of microbiome-based therapies’ potential in treating chronic diseases like inflammatory bowel disease, cancer, and neurological disorders. The market, focused on developing and manufacturing LBPs, live microorganisms designed for therapeutic purposes, is propelled by rising investments in R&D.
Additionally, key trends include advancements in microbial engineering and AI-driven manufacturing, enhancing precision and scalability. Regulatory support from agencies like the FDA and the growing prevalence of C. difficile infections fuel demand for specialised CDMOs. Partnerships between biopharma firms and CDMOs, alongside a shift toward personalised medicine, further accelerate growth.
Latest Market Trend
Advancements in Microbial Engineering and Ai-Driven Manufacturing
A significant trend in the live biotherapeutic products and microbiome CDMO market is the advancement of microbial engineering and AI-driven manufacturing, enhancing the precision and scalability of LBP production. Techniques like synthetic biology and CRISPR enable tailored microbial strains, while AI optimises fermentation and quality control, reducing production costs. The market is projected to grow with microbial engineering driving innovation.
- For example, in October 2024, OmniaBio Inc., a CDMO specialising in cell and gene therapies (which share significant manufacturing complexities with LBPs), opened its new commercial-ready manufacturing facility and AI centre of excellence in Hamilton, Ontario, Canada. This 100,000 sq. ft. facility is Canada's largest CDMO dedicated to CGTs and is explicitly designed for AI-enabled preclinical process, analytical development, and GMP manufacturing.
These advancements align with regulatory frameworks, such as the FDA’s LBP guidelines, and support scalable production, making this trend pivotal for market expansion in personalised medicine and chronic disease treatment.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 55.19 Million |
| Estimated 2025 Value | USD 76.77 Million |
| Projected 2033 Value | USD 1,075.98 Million |
| CAGR (2025-2033) | 39.10% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Lonza Group, WuXi Biologics, Thermo Fisher Scientific, Catalent, Bacthera |
to learn more about this report Download Free Sample Report
Live Biotherapeutic Products and Microbiome Cdmo Market Driver
Rising Prevalence of Chronic Diseases and Regulatory Support
The rising prevalence of chronic diseases and supportive regulatory frameworks are key drivers of the live biotherapeutic products and microbiome CDMO market. Conditions like C. difficile infections, IBD, and diabetes, with global cases exceeding 1 billion, increase demand for microbiome-based therapies. The market growth is driven by these health challenges.
- For example, in August 2024, tiny capsules of gut microbiota (“crapsules”) are being trialled in the NHS and private clinics in the UK to treat severe diseases like liver disorders, Parkinson’s, and cancers. FMT is currently approved in the UK for recurrent C. difficile, with ongoing studies expanding its application, highlighting regulatory recognition and increasing demand for microbiome therapies addressing chronic conditions.
Moreover, regulatory clarity and rising healthcare spending encourage biopharma to outsource to CDMOs, enhancing scalability and innovation in therapies targeting chronic and infectious diseases.
Market Restraining Factors
Manufacturing Complexity and Lack of Standardisation
The complexity of manufacturing live biotherapeutic products and the lack of standardised protocols pose significant restraints on the market. Producing LBPs requires strict control over microbial growth, purity, and consistency, which is difficult to maintain across large batches. There are also no globally accepted manufacturing standards for LBPs, making it harder for companies to ensure compliance across regions. This complexity increases production costs, delays clinical trials, and deters new players from entering the market.
Smaller biotech firms struggle due to the high costs of specialised manufacturing facilities and stringent quality requirements, particularly for conditions like C. difficile, which demand sterile environments. Moreover, regulatory frameworks differ between regions such as the U.S. and Europe, creating additional hurdles in global product approvals. These challenges collectively slow down scalability and innovation, particularly in developing regions, making industry-wide standardisation and infrastructure support essential to overcome these barriers.
Market Opportunity
Expansion of Personalised Medicine and Strategic Partnerships
The growing focus on personalised medicine presents a promising opportunity for the LBP and microbiome CDMO market. As treatments increasingly aim to match individual gut profiles and genetic data, microbiome-based therapies are gaining momentum. CDMOs are critical in helping biotech firms scale these personalised therapies, especially during clinical trials. Strategic collaborations are rising as companies seek partners to manage complex microbial production processes.
- For example, WuXi Biologics’ launch of a new microbial manufacturing facility in Chengdu with large-scale fermenters in 2025 highlights how infrastructure is expanding to support this trend.
Research institutions and government agencies also invest heavily in microbiome research, enhancing opportunities for CDMOs to offer precision manufacturing services. These partnerships not only accelerate innovation but also reduce time-to-market for new therapies.
Regional Insights
North America dominates the global LBP and microbiome CDMO market due to a strong biotech ecosystem, high R&D investment, and clear regulatory frameworks. The U.S. FDA’s well-defined LBP approval guidelines significantly streamline the development-to-market pathway. Companies like Finch Therapeutics and Seres Therapeutics drive innovation in oncology and recurrent C. difficile infections. Substantial funding from the NIH, alongside collaborations with CDMOs such as Bacthera, supports production scalability. Canada contributes through government innovation funds and facility expansions, especially in neurological and gastrointestinal applications. The region benefits from regulatory clarity, access to advanced technology, and a mature outsourcing culture, making it a global leader in personalised microbiome-based therapies.
U.s. Live Biotherapeutic Products and Microbiome Cdmo Market Trends
- The U.S. is the largest contributor to the global market, propelled by a mature biotech ecosystem, high volume of clinical trials, and strong regulatory support. The FDA’s clear guidance for LBP approvals has accelerated development pipelines. NIH investments and strategic partnerships are vital for CDMO capacity expansion. The focus on C. difficile and cancer therapies underscores the country’s leadership in targeted microbiome solutions. With a growing emphasis on personalised medicine, the U.S. remains a hotbed for innovation, making it a top outsourcing and development destination.
- Canada’s market is growing steadily due to strong governmental backing and a thriving biotech environment. Key drivers include the expanding domestic production capabilities, such as Lallemand Health Solutions’ Montreal facility upgrade for neurological LBP development. The country’s proximity to the U.S. and alignment with FDA standards streamline cross-border collaborations. Rising incidences of IBD and neurological conditions are spurring demand for specialised microbial therapies. Combined with Canada’s emphasis on high-quality, scalable contract manufacturing and its growing partnerships with U.S.-based microbiome firms, the country is carving a niche as a reliable CDMO hub.
Asia-Pacific Live Biotherapeutic Products and Microbiome Cdmo Market Trends
Asia-Pacific is the fastest-growing region, driven by expanding healthcare infrastructure, cost-efficient manufacturing, and strategic biopharma partnerships. China’s government-backed microbiome initiatives under the 14th Five-Year Plan and large-scale investments from firms like WuXi Biologics boost LBP production capacity. India, supported by DBT initiatives and a booming biotech sector, is rapidly emerging as a hub for microbial CDMO services. Partnerships such as Vedanta Biosciences’ 2024 tie-up with regional CDMOs highlight the region’s outsourcing potential. A surge in demand for therapies targeting antibiotic resistance and cancer, along with increasing clinical trials, positions Asia-Pacific as a vital region for scalable and affordable LBP development.
- China is leading the LBP and microbiome CDMO market in Asia-Pacific, driven by state-led investments and a robust biopharma ecosystem. Under the 14th Five-Year Plan, the government has allocated significant funding toward microbiome therapeutics, boosting domestic R&D. The oncology segment is expanding rapidly due to high cancer prevalence. Regulatory reforms and growing collaborations, such as those with Vedanta Biosciences, reinforce China’s role as a cost-effective and scalable manufacturing base for global and regional microbiome CDMO demand.
- India’s LBP and microbiome CDMO industry is expanding rapidly due to strong biotech growth, government incentives, and low-cost manufacturing. The Department of Biotechnology’s USD 10 million initiative in 2024 supports R&D in microbiome therapies. India's large patient base and rising concerns about antibiotic resistance are pushing demand for infectious disease-focused LBPs. Supported by its strength in biosimilars and growing collaborations with international firms like Ferring Pharmaceuticals, India is emerging as a global outsourcing hub for microbiome drug development and CDMO services.
Europe Live Biotherapeutic Products and Microbiome Cdmo Market Trends
Europe is experiencing significant growth in the LBP and microbiome CDMO market, driven by progressive regulatory frameworks and robust R&D in gut health and chronic disease management. Germany and the U.K. are at the forefront, hosting most clinical trials for gastrointestinal and neurological disorders. Initiatives like the EMA’s LBP guidelines and funding from national and EU-level programs foster innovation. Companies like Lonza and 4D Pharma are expanding microbial manufacturing capacities to support therapeutic development. Personalised medicine, supported by Horizon Europe and national microbiome R&D funds, continues to boost demand for CDMO services across the continent.
- Germany is a key player in Europe’s market, bolstered by advanced biopharma infrastructure and supportive regulations. The government’s EUR 50 million microbiome research fund and strong focus on gastrointestinal applications drive clinical activity. With support from the EMA and integration of Industry 4.0 technologies, Germany is well-positioned to deliver high-quality, specialised LBP solutions, especially in oncology and neurology, contributing significantly to the region’s CDMO landscape.
- The U.K. plays a prominent role in the European microbiome CDMO market, supported by favourable regulations and strong research funding. 4D Pharma’s expansion of its Leeds facility in 2025 to produce neurological LBPs reflects industry confidence. Participation in Horizon Europe and access to EUR 200 million in biotech funding enhance innovation. Collaborations with CDMOs like Lonza and alignment with EMA standards position the U.K. as a key player in advancing personalised microbiome therapies across Europe and beyond.
Component Insights
The solution segment, encompassing manufacturing equipment like fermenters, bioreactors, and analytical tools, dominates the market due to its critical role in scaling LBP production. The demand for advanced equipment is driven by the complexity of microbial manufacturing and the need for precision in clinical-grade therapeutics. For instance, WuXi Biologics’ microbial manufacturing campus in Chengdu, launched in June 2025, features a 15,000L fermenter, enhancing LBP scalability. North America’s 72% market share reflects heavy equipment adoption, driven by FDA regulatory support and biopharma outsourcing, ensuring the segment’s dominance in meeting the demand for high-quality LBPs.
Type Insights
Bacterial LBPs lead the type segment, holding an 80% share in 2024, due to their versatility in treating gastrointestinal disorders and infectious diseases. Their dominance is driven by advancements in microbial engineering, enabling tailored bacterial strains for therapeutic efficacy. Seres Therapeutics’ SER-109, approved for C. difficile in 2023, exemplifies this, with production scaled by Bacthera’s Swiss facility in 2024. The segment’s growth is fueled by increasing cases of antibiotic-resistant infections, with 2.8 million reported annually in the U.S. Regulatory clarity from the FDA and EMA further supports development, positioning bacterial LBPs as the cornerstone of microbiome therapeutics.
Application Insights
The gastrointestinal disorders segment commands the largest market share, at 87.11% in 2024, driven by the high prevalence of C. difficile infections and IBD, affecting over 1 million patients annually in the U.S. The segment’s growth is propelled by successful LBP therapies and regulatory approvals. Ferring Pharmaceuticals’ REBYOTA, presented at the American College of Gastroenterology in October 2023, targets recurrent C. difficile, boosting demand for CDMO services. The segment benefits from increased healthcare spending, with USD 18.6 billion allocated to U.S. microbiome R&D in 2024, and partnerships with CDMOs for scalable production, ensuring its dominance in addressing unmet medical needs.
Company Market Share
The global market is highly competitive, with players focusing on innovation, strategic alliances, and capacity expansion. Companies invest in microbial engineering and AI-driven manufacturing to meet regulatory and scalability demands. Mergers, acquisitions, and partnerships with biopharma firms enhance market reach, while R&D drives therapies for chronic diseases. These firms leverage global supply chains, regulatory expertise, and advanced facilities to address the growing demand for LBPs, ensuring robust market growth.
Seres Therapeutics: Seres Therapeutics is a leading player in the LBP and microbiome CDMO market, focusing on bacterial LBPs for gastrointestinal disorders like C. difficile. Its AI-driven manufacturing and partnerships with CDMOs like Bacthera drive growth. Seres’ FDA-approved SER-109, scaled in 2024, strengthens its market position. The company’s R&D investments and collaborations with biopharma firms align with personalised medicine trends, ensuring sustained growth.
Recent News
- In October 2023, Seres expanded its partnership with Bacthera to manufacture SER-109, establishing a dedicated facility in Switzerland’s Microbiome Center of Excellence, enhancing production capacity for C. difficile treatments and reinforcing its leadership.
List of Key and Emerging Players in Live Biotherapeutic Products And Microbiome CDMO Market
- Lonza Group
- WuXi Biologics
- Thermo Fisher Scientific
- Catalent
- Bacthera
- Seres Therapeutics
- Ferring Pharmaceuticals
- Exeliom Biosciences
- 4D Pharma
- Finch Therapeutics
- Vedanta Biosciences
- Hansen Holding
- Recipharm
- Evonik Industries
- Biocon

to learn more about this report Download Market Share
Recent Developments
- June 2025-WuXi Biologics launched a microbial manufacturing campus in Chengdu, China, featuring a 15,000L fermenter for LBP production. This facility supports gastrointestinal and oncology applications therapies, strengthening China’s position as a global CDMO hub and addressing Asia-Pacific’s growing demand.
- November 2024- Lonza expanded its microbial manufacturing capacity in Cologne, Germany, to scale LBP production for oncology and neurological disorders. The upgrade incorporates AI-driven analytics, enhancing batch consistency and supporting therapies like Exeliom’s EXL01, aligning with Europe’s regulatory demands.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 55.19 Million |
| Market Size in 2025 | USD 76.77 Million |
| Market Size in 2033 | USD 1,075.98 Million |
| CAGR | 39.10% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Component, By Type, By Application |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Live Biotherapeutic Products And Microbiome CDMO Market Segments
By Component
- Solution
- Services
By Type
- Bacterial LBPs
- Viral LBPs
- Fungal LBPs
- Others
By Application
- Gastrointestinal Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































