Monoclonal Antibodies Market Size, Share & Trends Analysis Report, By Type (Human, Humanized, Chimeric, and Murine), By Application (Oncology, Autoimmune Diseases, Hematologic diseases, Neurological Diseases, Infectious Diseases, Dermatology, Others), By Distribution Channel (Hospital Pharmacies, Drug Stores & Retail Pharmacies, and Others), and By Region (North America, Europe, Asia Pacific, Middle East & Africa, Latin America) Forecasts, 2025-2033
Monoclonal Antibodies Market Size
The global monoclonal antibodies market size was valued at USD 2,54,219.64 million in 2024 and is projected to grow from USD 2,92,683.07 million in 2025 to USD 9,16,719.19 million by 2033, exhibiting a CAGR of 15.2% during the forecast period (2025-2033).
The major factors contributing to the growth of monoclonal antibodies industry are the increasing prevalence of cancer and autoimmune diseases such as rheumatoid arthritis, psoriasis, Crohn's disease, and multiple sclerosis. In addition, technological advancements in genetic engineering and biomanufacturing, such as hybridoma techniques, have increased the efficiency of monoclonal antibodies, which makes them highly popular in cancer management. Furthermore, expanding application of approved monoclonal antibodies in oncology, immunology, and infectious diseases further contributes to the growing demand for monoclonal antibodies, which in turn, boosts the market growth.
- For instance, the graph below shows the prevalence of cancer in the U.S., highlighting the year and year growth of cancer cases from 2024 to 2025, which drives the need for effective monoclonal antibodies for its treatment.
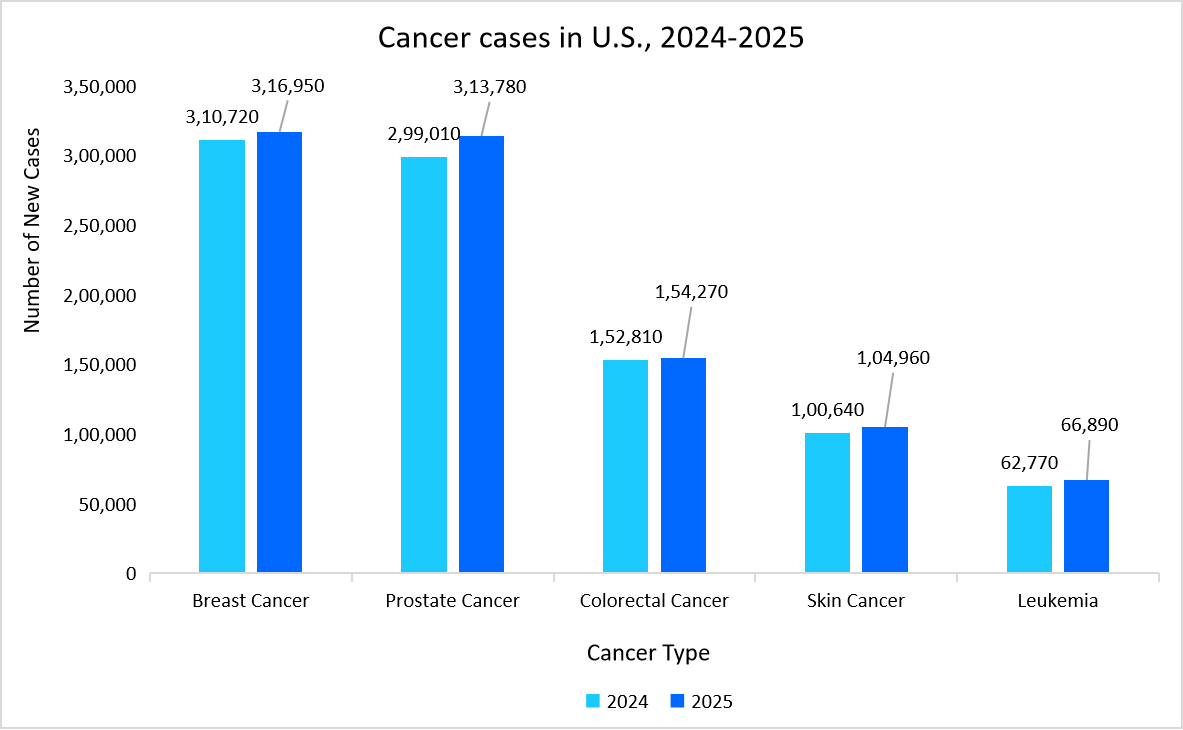
Source: American Cancer Society
Monoclonal Antibodies Market Trends
Expanding Indications beyond Oncology
An emerging trend in the monoclonal antibodies is growing approvals of previously approved monoclonal antibodies for expanding application, these applications include autoimmune diseases, neurological disorders, and rare diseases. For instance, In July 2024, the U.S. FDA approved Eli Lilly’s donanemab (Kisunla), a monoclonal antibody for early-stage Alzheimer’s, marking a significant advancement in therapeutic applications beyond oncology. This trend has driven the R&D activities for developing advanced monoclonal antibodies and also increased the demand for the management of various diseases.
- For example, the below table indicates the expanded approvals of Enhertu, a monoclonal antibody, to treat various diseases beyond cancer.
|
Expansion of Label and Indication of Enhertu (trastuzumab deruxtecan) |
|
|
Year of Approval |
Indication |
|
January 2025 |
Metastatic hormone receptor (HR)-positive, HER2-low breast cancer |
|
April 2024 |
HER2-positive solid tumours |
|
August 2022 |
Metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior chemotherapy in the metastatic setting |
|
May 2022 |
Metastatic HER2-positive breast cancer who have received prior anti-HER2-based regimen in the metastatic setting |
|
January 2021 |
Gastroesophageal junction (GEJ) adenocarcinoma |
Source: AstraZeneca and DAIICHI SANKYO COMPANY, LIMITED Press Release
Thus, such advancements are driving the demand for monoclonal antibodies to treat various diseases.
Rise of Precision Medicine and Biomarker-Based Therapies
Currently, new mAbs are being developed to bind to specific proteins such as PD-1, HER2, and EGFR. These drugs focus on treatments to individual patient profiles based on genetics. This improves the clinical outcomes, improving efficacy of the drugs products.
- For example, Trastuzumab is the only effective in patients whose tumors overexpress the HER2 receptor, this was identified through immunohistochemistry (IHC) testing.
- In August 2022, the FDA approved trastuzumab deruxtecan (Enhertu) for HER2-low breast cancer, a new molecular classification.
Thus, development of such targeted precision monoclonal antibodies is an emerging trend in the market.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 2,54,219.64 Million |
| Estimated 2025 Value | USD 2,92,683.07 Million |
| Projected 2033 Value | USD 9,16,719.19 Million |
| CAGR (2025-2033) | 15.2% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | AbbVie Inc., Amgen Inc., AstraZeneca plc, Biogen Inc., Bristol-Myers Squibb Company |

to learn more about this report Download Free Sample Report
Monoclonal Antibodies Market Growth Factors
Rising Demand for Monoclonal Antibodies to Treat Chronic Disease Conditions
The rise in the number of people suffering from various chronic diseases, such as cancer, autoimmune disorders, and other rare diseases are driving the need for monoclonal antibodies for effective disease management.
- For instance, the graph below highlights the global rise in number of people suffering from cancer and neurologic disorders, highlighting the need for monoclonal antibodies.
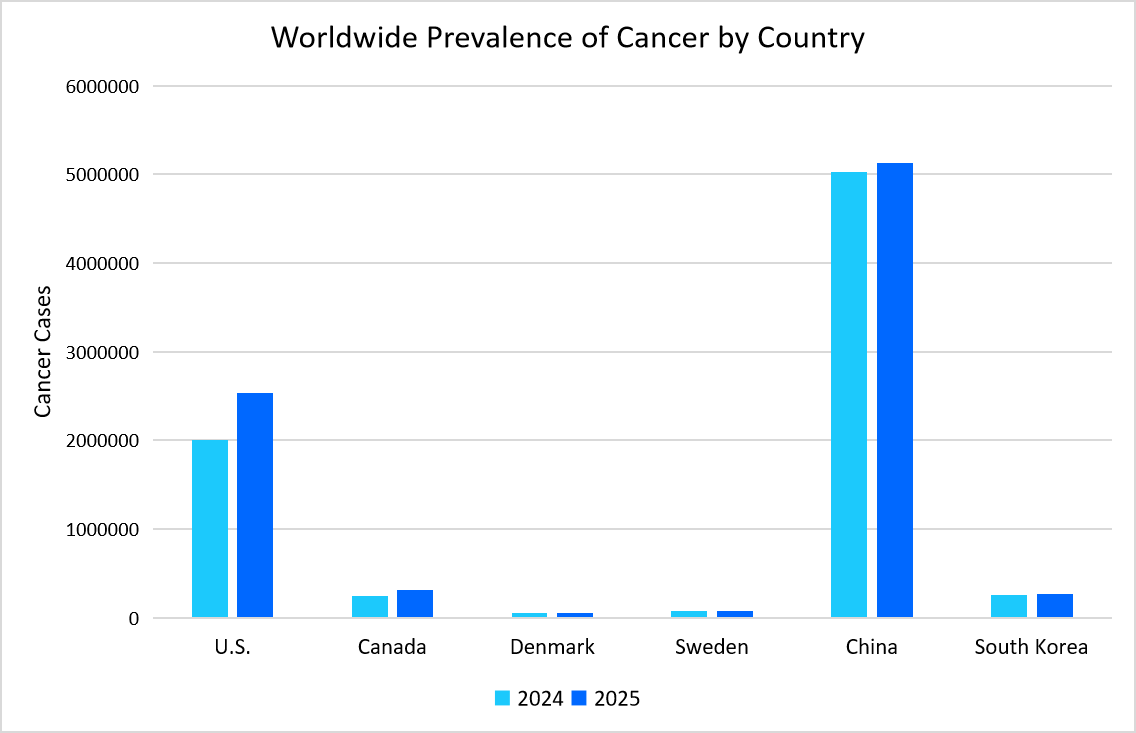
Source: World Health Organization & Straits Research
Further, growing incidence of rare diseases such as myasthenia gravis followed by early access authorization further drives the market growth. According to Myology 2022 conference, 20,000 people suffer from myasthenia gravis in France. In addition, ravulizumab (Ultomiris) received approval in May 2022, and efgartigimod (Vyvgart) in July 2022 for the rare disease management, both factors collectively support the market growth.
Thus, as the chronic disease burden is growing, so does the need for monoclonal antibodies rises for efficient disease management.
Growth in Year-on-Year Funding for Monoclonal Antibodies
Government and various organizations are actively providing funding for the development and procurement of monoclonal antibodies is boosting the confidence among manufacturers to invest in the development and production of monoclonal antibodies, which in turn supports the market growth. Thus, providing financial coverage for development and costly treatments helps reduce financial burden and encourages healthcare providers to prescribe these medications.
- For example, in September 2024, the University of Texas Medical Branch have been awarded with an amount of USD 15 million per year from National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH) to develop vaccines and monoclonal antibodies.
- In January 2025, Bill & Melinda Gates Foundation (BMGF) in partnership with LifeArc, which is a UK based medical research charity, announced to provide funding for sourcing and seeding innovations as well as accelerating the development of transformational solutions for reducing the costs of monoclonal antibody production.
- In November 2022, Torgny Stigbrand invests in Lipum, for financing the recently initiated phase I study with SOL-116, which is a humanized monoclonal antibody that blocks Bile Salt-Stimulated Lipase (BSSL), which is a previously overlooked target molecule in the immune system.
Market Restraining Factor
High Pricing of Monoclonal Antibodies Impacts Their Adoption
The high cost of monoclonal antibodies is unaffordable by low and middle-income countries also developed countries still facing financial burden due to high cost of the treatment. Thus, availability of alternative therapies for the treatment of these diseases are preferred, which impacts the adoption of monoclonal antibodies
- Below graph highlights the cost of few monoclonal antibodies between Switzerland and UK.
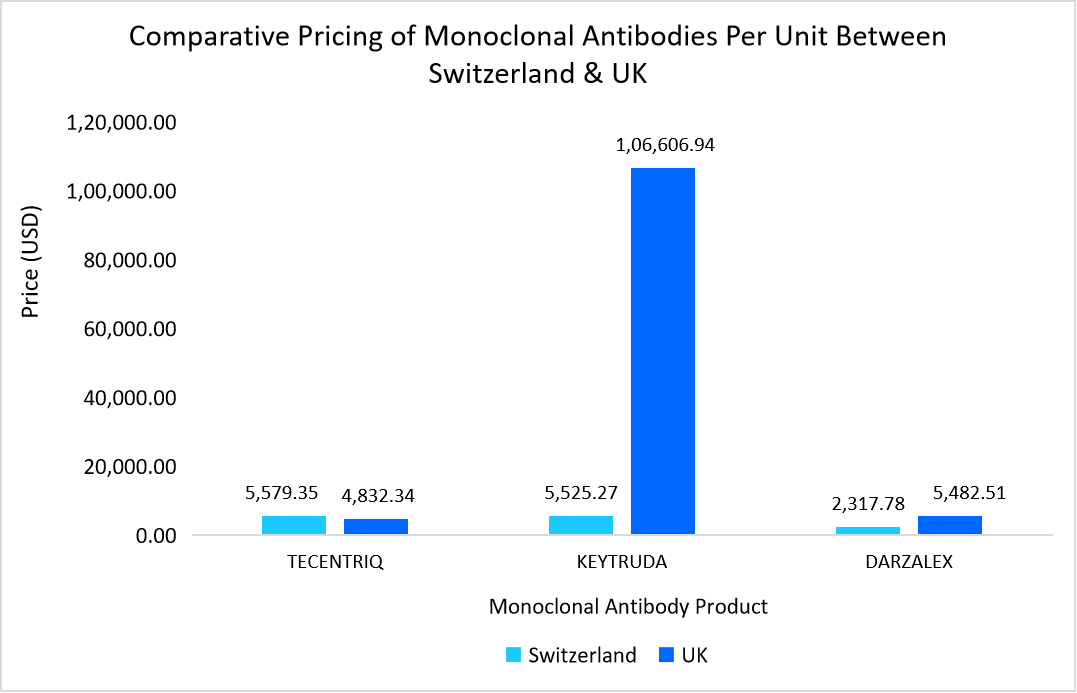
Source: Public Eye and National Institute for Health and Care Excellence
Market Opportunity
Clinical Trial Momentum: A Key Opportunity to Fuel Innovation
The increasing number of clinical trials for developing monoclonal antibodies presents a significant opportunity for the growth of monoclonal antibodies market during the forecast period.
- For example, below are the leading companies operating in the market for monoclonal antibodies have various candidates under pipeline for numerous disease indications.
Table 1: Monoclonal Antibodies Pipeline of U.S.-Based Companies
|
Company Name |
Product Name |
Development Phase |
Indication |
|
AbbVie Inc. |
Elezanumab (ABT-555) |
Phase-III |
Spinal Cord Injury |
|
AbbVie Inc. |
OpSCF |
Phase-III |
Atopic Dermatitis |
|
AbbVie Inc. |
ABBV-181 (budigalimab) |
Phase-III |
Solid Tumors |
|
AbbVie Inc. |
Livmoniplimab (ABBV-151) + Budigalimab (ABBV-181) |
Phase-III |
Liver, Solid Tumors, Non-Small Cell Lung Cancer |
|
AbbVie Inc. |
TTX-030 |
Phase-III |
Advanced Pancreatic Cancer |
|
AbbVie Inc. |
ABBV-141 |
Phase-II |
Idiopathic Pulmonary Fibrosis |
|
AbbVie Inc. |
ABBV-142 |
Phase-II |
Idiopathic Pulmonary Fibrosis |
|
Otsuka America Pharmaceutical, Inc |
Sibeprenlimab |
Investigational New Drug Application Submitted |
IgA Nephropathy |
|
Merck & Co., Inc. |
Clesrovimab (MK-1654) |
Under Review |
Respiratory Syncytial Virus |
|
Merck & Co., Inc. |
KEYTRUDA (MK-3475) |
Under Review |
1L Unresectable Advanced or Metastatic Malignant Pleural Mesothelioma |
|
Under Review |
Hepatocellular carcinoma (EU) |
||
|
Under Review |
Ovarian Cancer |
||
|
Under Review |
Small Cell Lung Cancer |
||
|
Phase- III |
Advanced Solid Tumors |
||
|
Phase- III |
Prostate Cancer |
||
|
Merck & Co., Inc. |
Tulisokibart (MK-7240) |
Under Review |
Crohn’s Disease |
|
Under Review |
Ulcerative Colitis |
||
|
Phase- III |
Systemic Sclerosis |
||
|
Merck & Co., Inc. |
Boserolimab (MK-5890) |
Phase-III |
Neoplasm Malignant |
|
Merck & Co., Inc. |
Quavonlimab (MK-1308) |
Phase-III |
Non-small Cell Lung Cancer |
|
Amgen Inc. |
Aimovig (erenumab-aooe) |
Phase-III |
Pediatric Migraine |
|
Amgen Inc. |
AMJEVITA (adalimumab-atto) |
Phase-III |
Interchangeability |
|
Amgen Inc. |
BEMARITUZUMAB |
Phase- III |
Gastric and Gastroesophageal Junction (GEJ) Cancers |
|
Phase- II |
Advanced Solid Tumors |
||
|
Amgen Inc. |
EVENITY (romosozumab-aqqg) |
Phase- III |
Male Osteoporosis |
|
Phase- III |
Pediatric Osteogenesis Imperfecta |
||
|
Amgen Inc. |
PROLIA (denosumab) |
Phase- III |
Pediatric Glucocorticoid-Induced Osteoporosis |
|
Amgen Inc. |
Repatha (evolocumab) |
Phase- III |
Hypercholesterolemia |
|
Amgen Inc. |
Rocatinlimab (formerly AMG 451 / KHK4083) |
Phase- III |
Atopic Dermatitis |
|
Phase- III |
Prurigo Nodularis |
||
|
Phase- II |
Asthma |
||
|
Amgen Inc. |
TEPEZZA (teprotumumab-trbw) |
Phase- III |
Thyroid Eye Disease |
|
Amgen Inc. |
TEZSPIRE (tezepelumab-ekko) |
Phase- III |
Severe Asthma |
|
Phase- III |
Chronic Rhinosinusitis with Nasal Polyps |
||
|
Phase- III |
Eosinophilic Esophagitis |
||
|
Phase- II |
Chronic Obstructive Pulmonary Disease |
||
|
Amgen Inc. |
UPLIZNA (inebilizumab-cdon) |
Phase- III |
IgG4-Related Disease |
|
Phase- III |
Myasthenia Gravis |
||
|
Phase- II |
Systemic Lupus Erythematosus with Nephritis |
||
|
Amgen Inc. |
DAXDILIMAB |
Phase- II |
Dermatomyositis and Anti-synthetase Inflammatory Myositis |
|
Phase- II |
Discoid Lupus Erythematosus |
||
|
Amgen Inc. |
ORDESEKIMAB (formerly AMG 714 / PRV-015) |
Phase- II |
Celiac Disease |
|
Amgen Inc. |
AMG 104 |
Phase-II |
Asthma |
|
Amgen Inc. |
AMG 329 (formerly HZN-1116) |
Phase-II |
Sjögren’s Disease |
|
Amgen Inc. |
AMG 355 |
Phase- I |
Solid Tumors |
|
Amgen Inc. |
AMG 691 |
Phase- I |
Asthma |
|
Amgen Inc. |
AMG 732 (formerly HZN-280) |
Phase- I |
Thyroid Eye Disease |
Source: AbbVie Inc., Otsuka America Pharmaceutical, Inc., Merck & Co., Inc., Amgen Inc.
Regional Insights
North America dominated the monoclonal antibodies market, accounting for the largest revenue share of 47.09%, owing to availability of major key players in this region, high accessibility to newly launched monoclonal antibodies, well-established reimbursement schemes, and increasing prevalence of chronic diseases such as cancer and autoimmune diseases.
U.s. Monoclonal Antibodies Market Trends
- U.S.- monoclonal antibodies industry in U.S. is dominantly growing with rising new cancer cases and prominent regulatory support of U.S. FDA, and presence of major market players. Furthermore, high number of people suffering from Alzheimer's disease potentiates the market growth with increase in demand for monoclonal antibodies as an effective treatment. According to 2024 reports, 6.9 million people from U.S. aged 65 and older are living with Alzheimer’s disease and it is estimated to reach 13.8 million by 2060.
Asia Pacific Monoclonal Antibodies Market Trends
Asia Pacific region is anticipated to grow at fastest CAGR of 16.4% during the forecast period. This growth is driven by increasing prevalence of cancer, arthritis, and other diseases, establishment of global pharmaceutical companies in India, and increasing awareness about the efficiency of monoclonal antibodies among healthcare providers.
- China – China’s monoclonal antibody market has witnessed significant growth, driven by advancements in biopharmaceutical research and regulatory improvements. Furthermore, strong development pipeline over 60 pharmaceutical companies are expected to enhance the treatment accessibility of monoclonal antibodies during the forecast period.
- For instance, the below graph highlights the China’s strong domestic 142 ongoing R&D projects.
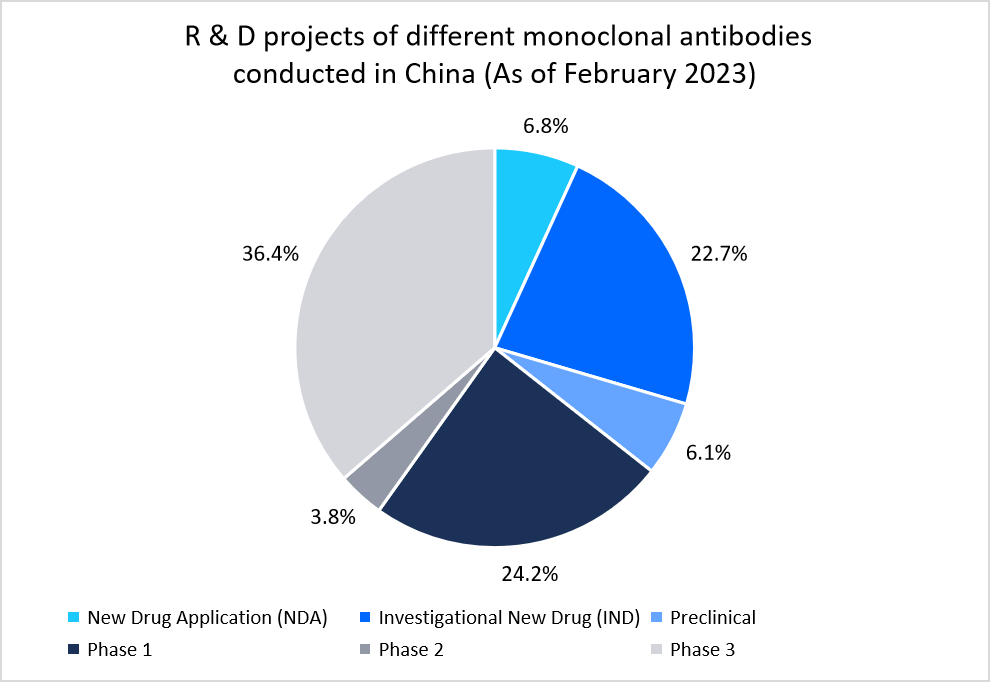
Source: Generics and Biosimilars Initiative (GaBI)
- India – India is witnessing strong growth in its monoclonal antibodies market, driven by increasing breast cancer cases. For example, in 2022, according to the Global Cancer Observatory, breast cancer was the leading causes of major cancer cases in India with 192,020 cases. This creates greater adoption of monoclonal antibodies used in treatment such as Herceptin (trastuzumab). Additionally, the increase in government funding for biotechnology research and development is driving the market growth.
- For example, in the union budget of 2024-2025 the government of India announced ₹90,171 core for healthcare sector and for research and development to boost local manufacturing and production in the country.
Europe Monoclonal Antibodies Market Trends
- UK – The major factors propelling the growth of monoclonal antibodies market in UK is the availability of the investments for the purpose of manufacturing monoclonal antibodies across the country and also owing to the high prevalence of severe diseases such as dementia as well as endometrial cancer, which require monoclonal antibodies for its treatments. For example, in 2022, as per the report of World Cancer Research Fund, total of 420,368 cases of endometrial cancer were witnessed across the globe, in which UK landed in the list of top 10 countries of endometrial cancer cases, which signifies the need for monoclonal antibodies for the treatment of such conditions.
- For example, the graph below depicts the cases of dementia across UK in the year 2022 and 2024, which reflects a significant increase among the UK’s population.
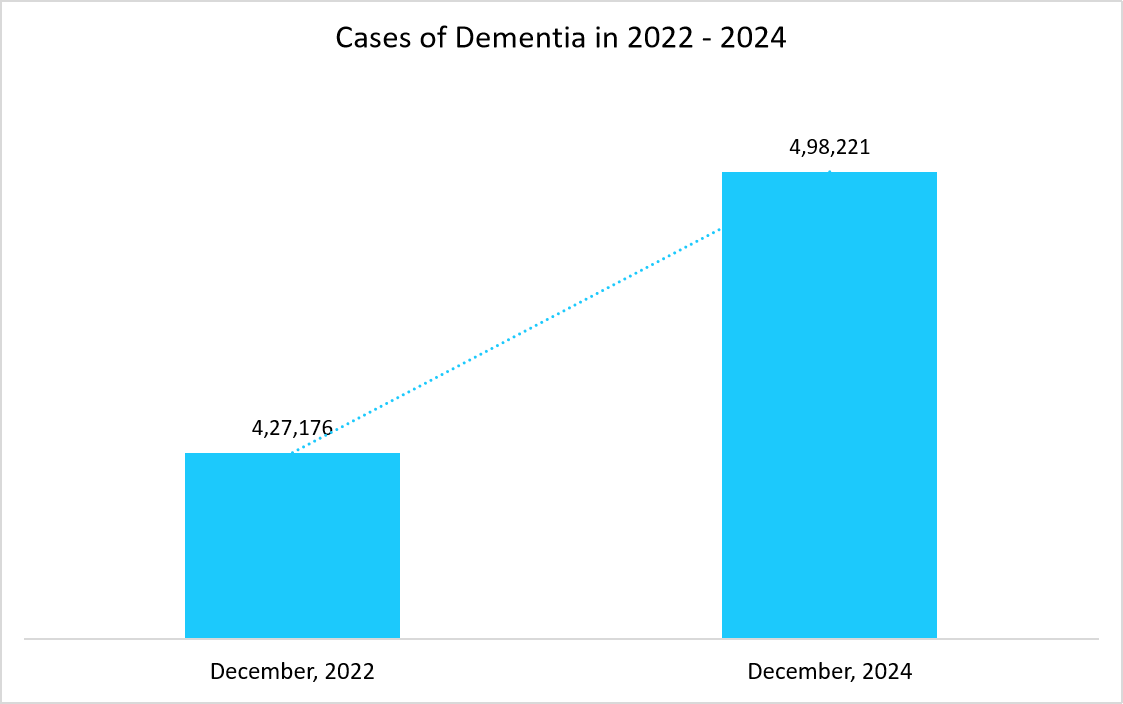
Sources: Nuffield Trust
- Germany – The Germany’s monoclonal antibodies industry is rapidly growing owing to is the focus of various research organizations such as German Centre for Infection Research towards the development, production and clinical testing of monoclonal antibodies. In addition, the market is also primarily driven by the high incidences of lung cancer as well as RSV infections across the country. For example, 3,911,800 cases per season of RSV were reported in Germany from the year 2022 to 2023, which reflects the growing need of monoclonal antibodies for the treatment of RSV infection.
- Spain– The Spain market is primarily driven by the high prevalence of asthma, availability of well-established production units and growing demand for monoclonal antibodies.
- For instance, the below graph highlights some of the most prescribed monoclonal antibodies for asthma are mentioned along with their percentage in the graph below.
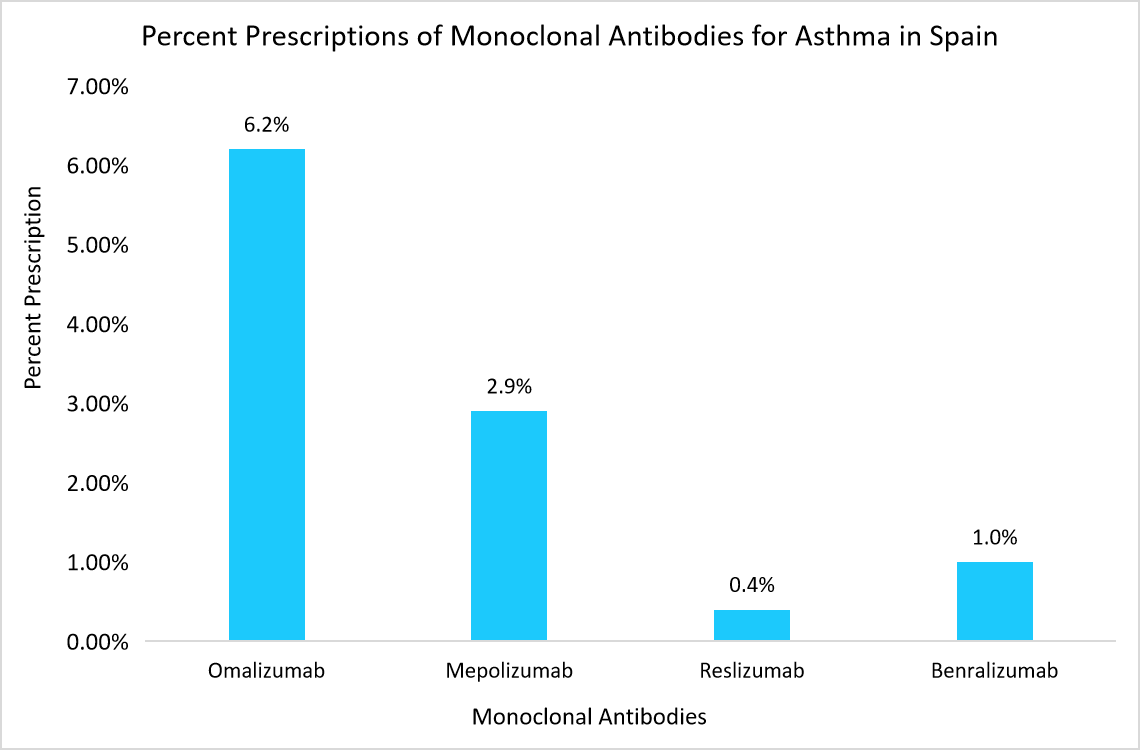
Source: National Institutes of Health(NIH)
Type Insights
The market is segmented into human, humanized, murine, and chimeric. Human segment accounted for the largest monoclonal antibodies market share, owing to increasing use of human monoclonal antibodies in cancer and autoimmune diseases, growing approvals of monoclonal antibodies, preference as first-line treatment for cancer, and increasing funding for development of human monoclonal antibodies.
Application Insights
The market is segmented into oncology, autoimmune diseases, hematologic diseases, neurological diseases, infectious diseases, dermatology, others. Oncology segment dominated the market and is expected to register fastest CAGR during the forecast period. This growth is driven by increasing incidences of cancer, use of monoclonal antibodies as a preferred treatment for cancer. Additionally, increasing approvals of monoclonal antibodies for cancer treatment further contribute towards the segment growth.
Distribution Channel Insights
The market is segmented into hospital pharmacies, drug stores & retail pharmacies, and others. Hospital pharmacies segment accounted for maximum revenue share owing to rising incidences of rheumatoid arthritis, colorectum cancer, and infectious diseases, which enhance the demand for new treatments, that lead to availability of monoclonal antibodies as a novel treatment via the hospital care. Furthermore, alliances between hospitals to promote cancer research and development, novel therapies availability with clinical expertise, also increases preference for hospital pharmacies by patients.
Company Market Share
Key players in the industry are majorly concentrated on the development of advanced monoclonal antibodies and are engaged into collaborations among key players for technology transfer to stay competitive in the market.
Accel-Heal Technologies Limited: An Emerging Player in the Monoclonal Antibodies Market
Almirall, S.A. is a Spanish pharmaceutical company emerge as an significant player, owing to its novel monoclonal antibody development and launch of new products.
Recent developments by Almirall, S.A.:
- In October 2024, Almirall launched lebrikizumab in Spain for the treatment of atopic dermatitis in adolescents and adults and in February 2024, it received the license from Novartis to develop anti-IL-21 monoclonal antibody in dermatology.
List of Key and Emerging Players in Monoclonal Antibodies Market
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Biogen Inc.
- Bristol-Myers Squibb Company
- Lilly
- Genmab A/S
- GlaxoSmithKline plc
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- Roche Holding AG
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- UCB S.A.
Recent Developments
- In November 2024, Eisai Co., Ltd. and Biogen Inc. launched monoclonal antibody, named as, LEQEMBI, in South Korea, for the treatment of adult patients with mild cognitive impairment due to Alzheimer's disease or mild AD dementia.
- October 2024: AGC Biologics received approval for all 2024 biologics applications including monoclonal antibodies from U.S. Food & Drug Administration (FDA).
- In February 2024, AbbVie and OSE Immunotherapeutics announced partnership in February 2024 for the development of novel monoclonal antibody for the treatment of chronic inflammation.
Analyst Opinion
The Monoclonal Antibodies market is witnessing significant growth, driven by the rising prevalence of chronic diseases, strong product pipeline, intellectual rights for approved products, increasing funding for research, surge in new product approvals, expanding application of monoclonal antibodies to treat various cancers, and improved reimbursement policies that make treatments more accessible.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 2,54,219.64 Million |
| Market Size in 2025 | USD 2,92,683.07 Million |
| Market Size in 2033 | USD 9,16,719.19 Million |
| CAGR | 15.2% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Monoclonal Antibodies Market Segments
By Type
- Human
- Humanized
- Chimeric
- Murine
By Application
- Oncology
- Autoimmune Diseases
- Hematologic diseases
- Neurological Diseases
- Infectious Diseases
- Dermatology
- Others
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































