Mosquito-Borne Infections Testing Market Size, Share & Trends Analysis Report By Application (Disease Surveillance and Monitoring, Clinical Diagnostics, Public Health Screening, Vaccine Development and Trials, Research and Epidemiology, Others (e.g., Veterinary Testing)), By Test Type (Rapid Diagnostic Tests (RDTs), Molecular Tests (e.g., RT-PCR, LAMP), Serological Tests (e.g., ELISA), Antigen Detection Tests, Others (e.g., Immunofluorescence Assays), By End-User), Hospitals and Clinics (Diagnostic Laboratories, Public Health Agencies, Research Institutions, Community Health Centres) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Mosquito-Borne Infections Testing Market Overview
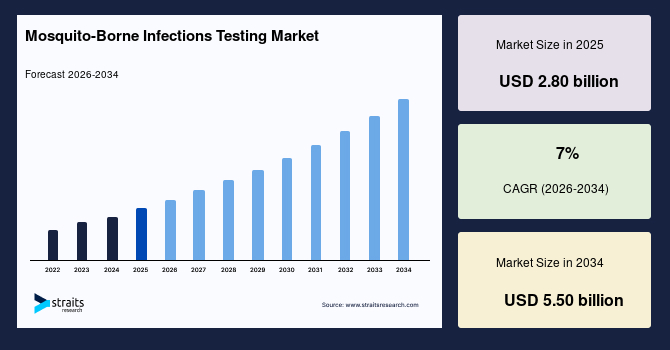
The global mosquito-borne infections testing market size was estimated at USD 2.80 billion in 2025 and is anticipated to grow from USD 3.00 billion in 2026 to USD 5.50 billion by 2034, growing at a CAGR of 7.0% from 2026-2034. The growth of the market is attributed to government initiatives and funding, Recurrent dengue outbreaks and expanding geographic range.
Key Market Trends & Insights
- Asia Pacific held a dominant share of the global market with a market share of 40% in 2025, owing to a large baseline incidence of dengue and malaria, high testing volumes and growing lab networks.
- The Latin America region is growing at the fastest pace, with a CAGR of 8.2%, due to explosive dengue outbreaks in 2024-2025 triggering emergency testing demand and expanded surveillance.
- Based on test type, the Rapid diagnostic tests (RDTs) dominated the market in 2025.
- Based on the end user, hospitals and clinics led in 2025.
- The U.S. dominates the mosquito-borne infections testing market in 2025.
Market Size and Forecast
- 2025 Market Size: USD 2.80 billion
- 2034 Projected Market Size: USD 5.50 billion
- CAGR (2026-2034): 7.0%
- Dominating Region: Asia Pacific
- Fastest-Growing Region: Latin America
The global mosquito-borne infections testing market growth is attributed to the rising prevalence of diseases like dengue, malaria, and Zika due to climate change and urbanisation, which demand rapid diagnostics. This is driven by increased global travel and vector spread into new regions, prompting investments in point-of-care testing.
The mosquito-borne infections testing market overview highlights key drivers such as surging disease outbreaks, with over 4 million dengue cases reported in 2025 according to the WHO, fueling demand for molecular and serological tests in endemic areas. The adoption of rapid antigen detection kits for field use and the integration of AI for predictive analytics in surveillance are market trends.
Market Trends
Rapid Scale-up of Ns1/igm Lateral Flow Testing for Outbreak Response
During the 2024–2025 dengue surges, field response relied heavily on rapid NS1 antigen and IgM/IgG combo lateral-flow tests for triage and case detection at primary care and emergency facilities. Vendors such as SD Biosensor and Abbott’s Bioline NS1 launched duo kits that had widespread field use for quick detection within minutes, enabling faster clinical decision-making and surveillance reporting.
- In April 2025, WHO's interim guidance recommended NS1/antibody rapid diagnostic tests (RDTs) for point-of-care testing in resource-limited and outbreak scenarios, which facilitates faster procurement and funding for these essential rapid diagnostic kits.
This created a large, recurring market for low-margin, high-volume rapid tests during epidemics, complemented by lab confirmation spend.
Rise of Molecular Diagnostics
Molecular techniques like RT-PCR and loop-mediated isothermal amplification (LAMP) dominate trends by offering high specificity for detecting Zika, chikungunya, and West Nile viruses, with detection limits as low as 10 copies per microliter. These methods enable multiplex testing for co-infections, vital in overlapping endemic zones, reducing false negatives by 30%. Portable devices facilitate use in low-resource settings, with battery-powered units operating in temperatures up to 40°C. AI-enhanced analysis speeds results to under 60 minutes, supporting vaccine trials and surveillance, helping market growth.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.80 billion |
| Estimated 2026 Value | USD 3.00 billion |
| Projected 2034 Value | USD 5.50 billion |
| CAGR (2026-2034) | 7% |
| Dominant Region | Asia Pacific |
| Fastest Growing Region | Latin America |
| Key Market Players | Abbott Laboratories, Thermo Fisher Scientific Inc, Roche Diagnostics, Bio-Rad Laboratories, Siemens Healthineers |
to learn more about this report Download Free Sample Report
Market Drivers
Government Initiatives and Funding
Government initiatives are a primary driver for the mosquito-borne infections testing market. As national and international health organisations grapple with rising cases, they are investing in diagnostic capacity to improve surveillance, accelerate response times, and prevent outbreaks.
- For instance, in January 2025, Brazil's Ministry of Health established the Public Health Emergency Operations Centre for Dengue and other Arboviruses (COE Dengue) to coordinate efforts against rising dengue cases by preparing health networks, expanding preventive measures, and strengthening national coordination.
Additionally, WHO and PAHO continue to issue alerts and guidance for dengue in the Americas. These fast-track evaluation and procurement pathways benefit vendors, encouraging investment in testing capacity.
Recurrent Dengue Outbreaks and Expanding Geographic Range
Epidemiologic data show that dengue incidence surged in 2024, with over 14 million reported globally as per the WHO. The Americas alone had more than 13 million suspected cases in 2024, driving exceptional testing demand for case management and surveillance.
- The WHO-declared dengue emergency and activation of diagnostic review mechanisms in 2025 accelerated procurement of tests and harmonised testing algorithms.
Outbreak-driven demand is large with countries scaling up rapid tests, PCR reagents and lab services rapidly during peaks, producing a significant revenue bump for diagnostics suppliers and recurring replacement demand each season.
Market Restraint
Cross-Reactivity in Serological Testing
A key limitation in mosquito-borne infection diagnostics is the serological cross-reactivity among flaviviruses. For example, antibodies for the dengue virus (IgM/IgG) often react with the Zika virus, the yellow fever virus, or antibodies from previous dengue infections. This overlap reduces the diagnostic specificity of serological tests, especially in regions where multiple flaviviruses circulate or where vaccination campaigns have been conducted. As a result, serological assays alone have limited reliability for case confirmation. Molecular tests (e.g., PCR) are increasingly relied upon for accurate diagnosis and serotyping, but these are more expensive and require well-equipped laboratories.
Market Opportunity
Integrated Point-of-Care (poc) Testing with Digital Reporting
Point-of-care antigen and antibody rapid tests have revolutionised outbreak response by enabling fast, on-site results, crucial for remote areas lacking labs. This model was widely piloted during the 2024–2025 dengue emergencies, with ministries of health and NGOs combining rapid kit distribution with centralised reporting dashboards to map cases and direct vector control resources. Companies like SD Biosensor, with their STANDARD Q dengue kits, have been extensively deployed in Asia and Latin America during this period. These integrated approaches not only generate higher-value revenue streams for suppliers but also encourage long-term procurement contracts from governments and donors.
Regional Analysis
Asia Pacific: Dominant Region with 40% Market Share
Asia Pacific is the largest volume region due to endemic transmission in South East Asia, South Asia, parts of the Western Pacific, and frequent seasonal and large outbreaks. Governments in India, China and Southeast Asian countries routinely allocate RDTs and laboratory kits through national programmes. The region’s dual demand geometry from rural/POC need for RDTs and urban reference-lab demand for PCR/NGS drives a mixed market where global vendors coexist with strong local manufacturers like J Mitra in India, SD Biosensor in Korea, etc. For suppliers, Asia Pacific represents both the highest unit volumes in rapid tests and a growing high-value lab business as governments invest in molecular and genomic surveillance infrastructure.
Latin America: Fastest-Growing Region with Significant Market Share
Latin America exhibits the fastest growth in the mosquito-borne infections testing market, with an 8.2% CAGR, driven by surging dengue and Zika cases, with Brazil reporting 6 million dengue cases in 2024 as per the WHO. Urban sprawl and climate shifts expand mosquito ranges, boosting test demand in the region. Government focus on outbreak preparedness and WHO-backed surveillance drives robust market expansion, addressing regional challenges like tourism-driven disease spread and making Latin America the fastest-growing region for diagnostic testing.
Countries Insights
U.s. Market Trends
The U.S. market is shaped by advanced diagnostic infrastructure and regulatory oversight. While most mosquito-borne cases are travel-related, locally acquired dengue in Florida, California, and Texas in 2024–2025 increased testing demand. The CDC expanded guidance for public health labs, recommending multiplex PCR assays to improve outbreak monitoring. Hospitals and reference labs rely on automated systems like Roche’s cobas for high-throughput testing, while clinics use NS1/IgM rapid tests for initial screening. Federal investment in genomic surveillance has further strengthened the detection of emerging pathogens such as Zika and West Nile virus.
Canada Market Growth Factors
Canada’s testing market is smaller in volume but highly regulated and focused on accuracy. Most mosquito-borne infections are travel-related, with screening concentrated in provincial public health labs and hospital systems. In 2025, the Public Health Agency of Canada and CATMAT linked diagnostics to dengue vaccination guidance, raising demand for reliable serological tests to confirm past exposure. Provincial labs such as Public Health Ontario maintain centralised PCR and ELISA testing, ensuring consistent results across regions.
China Market Trends
China is expanding its mosquito-borne infections testing market by combining community-based rapid testing with advanced molecular laboratories in major cities. The Chinese CDC has strengthened surveillance following dengue outbreaks in southern provinces, while hospitals in urban areas are adopting PCR and sequencing for accurate detection. The government supports local diagnostic kit manufacturing to reduce reliance on imports, which makes tests more affordable and widely available. China is a key dual-market for low-cost RDTs and higher-value molecular platforms.
Indonesia Market Growth Factors
Indonesia faces a high burden of mosquito-borne diseases, particularly dengue, with millions of cases reported each year. The government has prioritised diagnostic expansion as part of its national vector control programme. Rapid diagnostic tests remain critical in community health centres, while urban hospitals rely on PCR for confirmatory testing and co-infection detection. International donors, including WHO and the World Mosquito Program, also support surveillance upgrades, reinforcing Indonesia’s role as a key testing market in Southeast Asia.
Brazil Market Trends
Brazil is the largest testing market in Latin America, driven by recurring dengue outbreaks. In 2024, the country reported more than six million dengue cases, creating urgent demand for rapid testing. Urban hospitals increasingly use PCR platforms for confirmatory diagnostics, while RDTs remain essential in community health centres. Government partnerships with international donors and local manufacturers have helped distribute affordable kits widely. Brazil continues to scale up both rapid and advanced diagnostics to control outbreaks effectively.
Market Segmentation
Test Type Insights
Rapid diagnostic tests (RDTs) represent the leading segment. They are widely used due to their portability, low cost (USD 5–10 per test), and ability to provide results in under 30 minutes. These tests are essential in outbreak settings, particularly in regions with limited laboratory capacity. RDTs, such as NS1 antigen assays, demonstrate high sensitivity (about 95%), allowing for early case detection and reducing onward transmission by an estimated 20%. Their adoption is strongest in endemic regions of Africa and Asia, where large populations rely on point-of-care diagnostics.
End-User Insights
Hospitals and clinics hold the largest market share. These facilities manage high patient volumes and are equipped to conduct confirmatory testing for infections such as dengue, Zika, and chikungunya. Molecular and serological platforms, such as Roche’s cobas analysers, enable high-throughput testing, processing up to 1,000 samples daily. The rise of urban outbreaks linked to Aedes mosquito expansion has further increased the demand for multiplex assays that can detect co-infections. Trained laboratory staff and integrated workflows support this segment’s growth, ensuring accurate results during seasonal and travel-associated outbreaks.
Technology Insights
Molecular diagnostics, particularly reverse transcription polymerase chain reaction (RT-PCR), lead the market. These tests achieve about 98% accuracy, detecting even low viral loads. RT-PCR is valuable for differential diagnosis in cases of co-infection and can simultaneously identify multiple pathogens. Expanding investment in PCR capacity also creates ongoing demand for reagents, making it a high-value segment for suppliers. National programmes and international donors increasingly prioritise PCR platforms to enhance surveillance and improve early outbreak detection.
Competitive Landscape
The global market is highly fragmented and dominated by companies offering diverse diagnostics like PCR and RDTs, with strong R&D in multiplex and POC solutions to address outbreaks. Players leverage partnerships with health agencies for global distribution, focusing on affordability in emerging markets and innovation for high specificity, driving share through regulatory approvals and scalability amid climate-driven demands.
Roche Diagnostics: An Emerging Player
Roche Diagnostics pursues a platform-led strategy. Their automated immunoassay menus (Elecsys) and high-throughput molecular systems (cobas 6800/8800) sell instruments and recurring reagents to reference labs and national programmes. During the 2024–2025 dengue response, Roche emphasised expanded dengue assay coverage (antigen/Ig panels) and multiplex molecular workflows, aligning with WHO guidance for confirmation and surveillance.
Latest news:
- In May 2025, Roche included Elecsys Dengue Ag and expanded infectious-disease assay messaging in its Diagnostics Day material, signalling product-menu expansion for automated antigen testing and integrated lab workflows.
List of Key and Emerging Players in Mosquito-Borne Infections Testing Market
- Abbott Laboratories
- Thermo Fisher Scientific Inc
- Roche Diagnostics
- Bio-Rad Laboratories
- Siemens Healthineers
- Quest Diagnostics
- Danaher Corporation
- NovaTec Immundiagnostica GmbH
- InBios International Inc.
- Euroimmun AG
- DiaSorin S.p.A.
- Qiagen N.V.
- PerkinElmer Inc.
- OraSure Technologies
- Hologic Inc
- Becton Dickinson
Recent Developments
- August 2025- Roche Diagnostics received FDA clearance for cobas Respiratory 4-panel, adaptable for arboviruses like dengue, boosting high-throughput surveillance.
- June 2025 - QIAGEN announced a QIAcuity digital PCR IVD assay development partnership with Gencurix, underscoring moves into higher-precision molecular platforms that can be applied to arbovirus detection and research.
- July 2025 - SD Biosensor publicly exhibited its STANDARD Q rapid test portfolio at IAS 2025, signalling continued market emphasis on high-sensitivity lateral-flow kits for field use and outbreak response.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.80 billion |
| Market Size in 2026 | USD 3.00 billion |
| Market Size in 2034 | USD 5.50 billion |
| CAGR | 7% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Application, By Test Type, Hospitals and Clinics |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Mosquito-Borne Infections Testing Market Segments
By Application
- Disease Surveillance and Monitoring
- Clinical Diagnostics
- Public Health Screening
- Vaccine Development and Trials
- Research and Epidemiology
- Others (e.g., Veterinary Testing)
By Test Type
- Rapid Diagnostic Tests (RDTs)
- Molecular Tests (e.g., RT-PCR, LAMP)
- Serological Tests (e.g., ELISA)
- Antigen Detection Tests
- Others (e.g., Immunofluorescence Assays)
- By End-User
Hospitals and Clinics
- Diagnostic Laboratories
- Public Health Agencies
- Research Institutions
- Community Health Centres
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































