Multiplex Testing Reagents Market Size, Share & Trends Analysis Report By Type (Biotin-based Reagents, Streptavidin-based Reagents), By Application (Infectious Disease Diagnostics, Autoimmune Disease Diagnostics, Cancer Diagnostics, Drug Discovery and Development, Allergy Testing, Others), By End Use (Clinical & Diagnostic Laboratories, Pharmaceutical & Biotechnology Companies, Contract Research Organizations, Academic & Research Institutes, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Multiplex Testing Reagents Market Overview
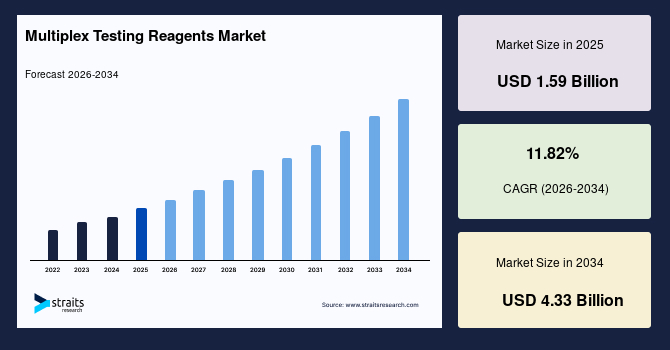
The global multiplex testing reagents market size is valued at USD 1.59 billion in 2025 and is estimated to reach USD 4.33 billion by 2034, growing at a CAGR of 11.82% during the forecast period. The consistent market growth is fuelled by the growing integration of microfluidic nano reagent systems for ultra-miniaturized multiplexing with unprecedented assay precision for diagnostic platforms.
Key Market Trends & Insights
- North America held a dominant revenue share of the global market, accounting for 37.46% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.18%.
- Based on type, the biotin-based reagents segment held the highest market share in 2025.
- By application, the cancer diagnostics segment is expected to register the fastest CAGR growth of 12.26%.
- Based on end use, the pharmaceutical & biotechnology companies segment dominated the market in 2025 with a revenue share of 37.95%.
- The U.S. dominates the market, valued at USD 480.29 million in 2024 and reaching USD 534.99 million in 2025.
Table: U.S. Multiplex Testing Reagents Market Size (USD Million)
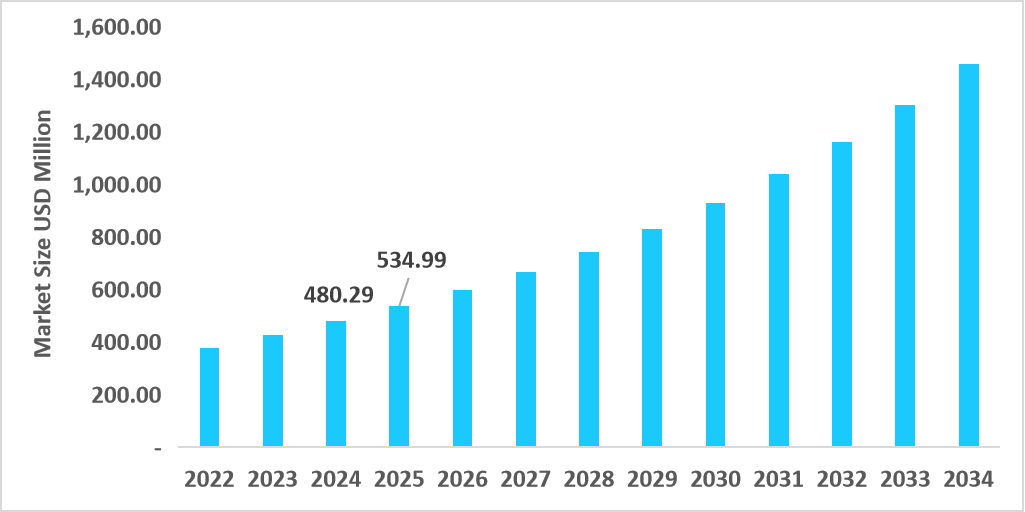
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.59 billion
- 2034 Projected Market Size: USD 4.33 billion
- CAGR (2026-2034): 11.82%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The global market includes a wide range of biotin-based and streptavidin-based reagents, including biotinylated primers, tagged proteins, antibodies, and streptavidin-conjugated enzymes, fluorophores, and beads. These reagents support diverse applications such as infectious and autoimmune disease diagnostics, cancer testing, drug discovery and development, and allergy assessment across clinical laboratories, pharmaceutical and biotechnology companies, CROs, and academic research institutes.
Latest Market Trends
Rising Adoption of High-Sensitivity Digital Multiplexing Platforms
A major trend in the multiplex testing reagents market is the rapid shift toward high-sensitivity digital multiplexing technologies that enable ultra-precise quantification of multiple biomarkers in a single reaction. These platforms utilize advanced bead-based and microchip-based chemistries to enhance signal clarity, lower detection limits, and improve assay reproducibility. Recent innovations in digital reagent design are supporting larger analyte panels, streamlined workflows, and expanded clinical utility. This trend underscores growing demand for more accurate and scalable diagnostic solutions.
Shift from Conventional Single-Analyte Assays to Integrated Multi-Omics Multiplexing
The shift from traditional single-analyte testing toward integrated multi-omics multiplexing platforms is a key trend for market growth. Leading diagnostic developers are forming strategic collaborations with genomics and proteomics technology companies to co-create reagents capable of simultaneously analyzing DNA, RNA, proteins, and metabolites within unified workflows. Such partnerships are accelerating the transition to more comprehensive diagnostic solutions that enhance research precision.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.59 Billion |
| Estimated 2026 Value | USD 1.77 Billion |
| Projected 2034 Value | USD 4.33 Billion |
| CAGR (2026-2034) | 11.82% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Abbott Laboratories, Abcam plc, Agilent Technologies, BD, bioMérieux |
to learn more about this report Download Free Sample Report
Market Drivers
Surging Demand for High-Throughput Disease Diagnostics
A notable driver in the multiplex testing reagents market is the rising global demand for high-throughput diagnostic solutions capable of detecting multiple biomarkers simultaneously. Healthcare systems are increasingly adopting multiplex assays to improve testing efficiency, reduce sample volume requirements, and accelerate clinical decision making across infectious disease, oncology, and autoimmune diagnostics. For instance, leading diagnostic networks such as Labcorp expanded their use of multiplex molecular panels to enhance rapid pathogen detection. Therefore, the push for faster, more comprehensive testing is propelling market growth.
Market Restraints
High Cost of Specialized Multiplex Reagents Limits Broader Adoption
The high cost associated with advanced multiplex assay reagents and platform-specific consumables is a key factor restraining the market growth. For example, the cost of the MILLIPLEX MAPK/SAPK 10‑Plex Kit is around USD 3,400 to 3,500. These expenses often exceed the budgets of smaller laboratories, particularly in emerging regions. Several mid-scale diagnostic centers in Latin America postponed adopting high-plex immunoassay reagents due to high kit prices and ongoing calibration costs. Thus, affordability challenges continue to hinder the widespread implementation of multiplex testing solutions.
Market Opportunities
Growing Expansion of Multiplex Testing in Decentralized and Point-of-Care Diagnostic Settings
A major opportunity in the multiplex testing reagents market is the accelerating transition from centralized laboratory diagnostics to decentralized and point-of-care testing environments. Various urgent care centers and community clinics across Asia and Europe have recently adopted portable multiplex PCR platforms from companies like BioFire and Cepheid to meet rising demand for rapid, multi-pathogen detection. This shift allows reagent manufacturers to reach broader end users, improve accessibility, and capitalize on the growing demand for fast, actionable diagnostic solutions across diverse healthcare settings.
Regional Analysis
North America dominated the market in 2025, accounting for 37.46% market share, driven by strong public-private funding collaborations supporting multi-omics biomarker research. Initiatives such as the NIH Common Fund enable large-scale multiplex projects, enhancing reagent adoption in clinical and research laboratories and strengthening the region’s position as a global leader in advanced diagnostics.
The U.S. multiplex testing reagents market is experiencing unique growth due to BARDA-backed initiatives supporting multiplex diagnostic development. By funding multi-pathogen assay programs for biodefense and pandemic preparedness, these efforts accelerate innovation, expand reagent adoption in clinical and research laboratories, and reinforce the U.S. as a leader in advanced, high-throughput diagnostic technologies.
Asia Pacific Market Insights
Asia Pacific is the fastest-growing region with a CAGR of 13.18% from 2026-2034. The growth is attributed to rising government initiatives in countries like China, Japan, and South Korea to expand national precision medicine programs, driving widespread adoption of multiplex testing reagents in clinical diagnostics and research laboratories.
Australia's multiplex testing reagents market is experiencing strong growth during the adoption of Genetic Signatures’ 3base technology, which streamlines multiplex pathogen detection into a single assay. Its integration across clinical and research laboratories enhances diagnostic efficiency, increases demand for multiplex testing reagents, and strengthens Australia’s position in advanced infectious disease diagnostics.
Regional Market share (%) in 2025
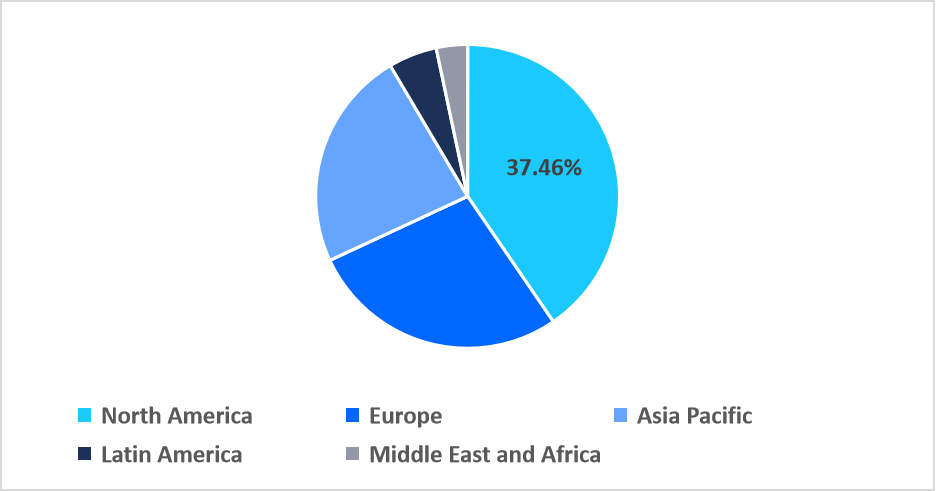
Source: Straits Research
Europe Market Insights
Europe is witnessing strong growth in the multiplex testing reagents market, driven by Horizon Europe-funded initiatives, such as the hmqPro project, which develops ultra-multiplex quantitative protein biomarker platforms. These programs boost demand for multiplex testing reagents in clinical research and pharmaceutical development, strengthening Europe’s leadership in advanced biomarker discovery.
Germany’s market growth is driven by the German Network for Bioinformatics Infrastructure, which enhances national bioinformatics capabilities. By supporting the development and integration of high-plex multiplex assays and advanced data analysis tools, de.NBI strengthens demand for multiplex testing reagents across research institutions and clinical laboratories in Germany.
Latin America Market Insights
The Latin America multiplex testing reagents market is benefiting from government-supported infectious disease screening programs in Brazil, which integrate multiplex assays into public health laboratories. This approach is driving the demand for multiplex testing reagents across the region.
The Argentine market growth is driven by the rapid expansion of the predictive biomarker diagnostics sector. This trend increases the adoption of multiplex testing reagents for personalized cancer diagnostics and translational research and supports the country’s advancement in precision medicine initiatives.
Middle East and Africa Market Insights
The Middle East and Africa market is being driven by accelerated regulatory approval of multiplex diagnostics in the Gulf region. For example, Saudi Arabia’s SFDA approval of BGI Genomics’ multiplex pathogen detection kits enhances adoption in clinical laboratories, expanding diagnostic capabilities and increasing demand for advanced multiplex testing reagents across the region.
The Saudi Arabia multiplex testing reagents market is benefiting from digital pathology-integrated multiplex testing in leading tertiary hospitals. By combining high-resolution imaging with multiplex assays, these facilities enhance simultaneous biomarker analysis and create a unique, technology-driven demand for advanced reagents in the region.
Type Insights
The biotin-based reagents segment dominated the market in 2025. This dominance is attributed to increasing adoption of ultra-stable biotin linkers engineered for high-temperature multiplex assays, allowing superior performance in complex thermal cycling workflows and expanding their applicability in molecular diagnostics.
The streptavidin-based reagents segment is projected to grow at a CAGR of 12.37% during 2026-2034, owing to rising utilization of engineered streptavidin variants with enhanced binding valency, enabling higher analyte loading capacity in bead-based multiplex platforms, and improving assay depth for complex biomarker panels.
Application Insights
The drug discovery and development segment dominated the market with a revenue share of 28.04% in 2025. This dominance is augmented by growing reliance on multiplex reagents for simultaneous off-target toxicity screening, enabling early identification of multi-pathway drug interactions and notably accelerating preclinical candidate validation with higher analytical efficiency.
The cancer diagnostics segment is anticipated to register the fastest CAGR of 12.26% during 2026-2034. This strong growth is driven by increasing deployment of multiplex reagents optimized for ultra-rare mutation detection in circulating tumor DNA, enabling highly sensitive liquid biopsy assays that support earlier cancer identification.
By Application Market Share (%), 2025
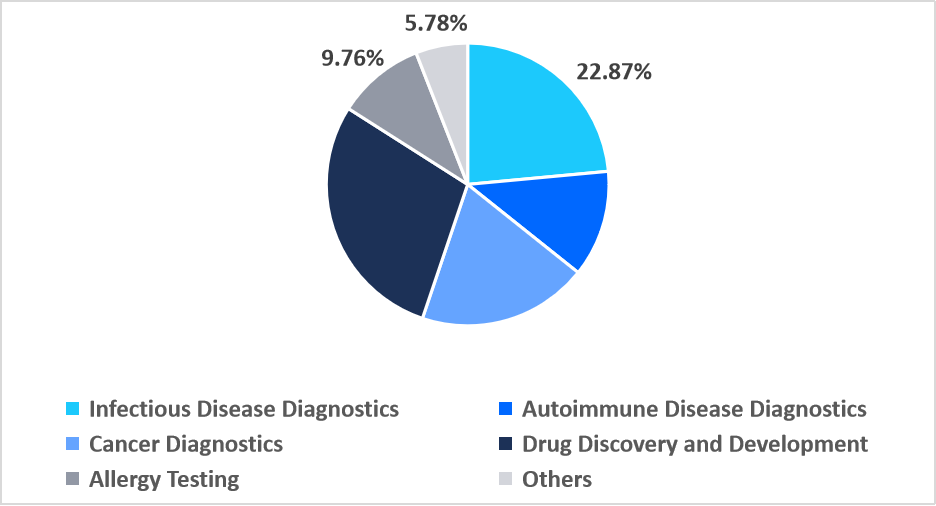
Source: Straits Research
End Use Insights
The pharmaceutical & biotechnology companies segment dominated the market with a revenue share of 37.95% in 2025. As these organizations increasingly depend on multiplex reagents to standardize multi-analyte quality control testing for biologics manufacturing, they must ensure consistent product characterization and regulatory compliance across large-scale production pipelines.
The clinical & diagnostic laboratories segment is expected to register the fastest CAGR growth during the forecast timeframe, as these laboratories increasingly adopt multiplex testing reagents to expand their service offerings, enhance multi-disease screening capabilities, and meet growing demand for comprehensive, cost-effective, and high-volume patient diagnostics.
Competitive Landscape
The global multiplex testing reagents market is consolidated, with a few multinational companies holding the majority of the market share. Leading players include Thermo Fisher Scientific, Bio‑Rad Laboratories, Luminex, Merck KGaA, BD Biosciences, Qiagen, Meso Scale Diagnostics, Agilent Technologies, and others. These companies are actively pursuing strategies such as launching innovative highplex assays, integrating digital platforms, forming strategic partnerships, and expanding into emerging markets, thereby driving growth and strengthening their global footprint.
Seegene Inc.: An emerging market player
Seegene, a South Korean molecular diagnostics company, is rapidly emerging as a key player in the market. Its proprietary technologies, DPO, TOCE, and MuDT, enable real-time PCR assays that detect up to 25 analytes per reaction using just five fluorescent channels.
- In August 2024, Seegene developed a 4‑plex MPXV/OPXV assay and other high multiplex viral panels in response to the WHO’s mpox health emergency.
List of Key and Emerging Players in Multiplex Testing Reagents Market
- Abbott Laboratories
- Abcam plc
- Agilent Technologies
- BD
- bioMérieux
- Bio-Rad Laboratories
- Bio-Techne Corporation
- Illumina, Inc.
- Luminex Corporation
- Merck KGaA
- Meso Scale Diagnostics (MSD)
- Olink Proteomics
- PerkinElmer Inc.
- Promega Connections
- QIAGEN N.V.
- Hoffmann-La Roche Ltd.
- Seegene Inc.
- Siemens Healthineers
- Thermo Fisher Scientific
- Others
Strategic Initiative
- November 2024: Augurex Life Sciences Corp. launched the Anti-14-3-3eta Multiplex Analyte Specific Reagents for supporting the development of the diagnosis of axial spondyloarthritis.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.59 Billion |
| Market Size in 2026 | USD 1.77 Billion |
| Market Size in 2034 | USD 4.33 Billion |
| CAGR | 11.82% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Multiplex Testing Reagents Market Segments
By Type
-
Biotin-based Reagents
- Biotinylated Primers
- Biotin-tagged Proteins/enzymes
- Biotinylated Antibodies
-
Streptavidin-based Reagents
- Streptavidin-conjugated Enzymes & Fluorophores
- Streptavidin-conjugated Beads
- Others
By Application
- Infectious Disease Diagnostics
- Autoimmune Disease Diagnostics
- Cancer Diagnostics
- Drug Discovery and Development
- Allergy Testing
- Others
By End Use
- Clinical & Diagnostic Laboratories
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Academic & Research Institutes
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































