Non-invasive Prenatal Testing Market Size, Share & Trends Analysis Report By Product (Instrument, Consumables, Services), By Application (Trisomy, Microdeletion Syndrome, Others), By End User (Hospitals, Clinics, Diagnostic Laboratories) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Non-invasive Prenatal Testing Market Overview
The global non-invasive prenatal testing market size is valued at USD 5.37 billion in 2025 and is estimated to reach USD 16.94 billion by 2034, growing at a CAGR of 13.67% during the forecast period. The accelerated growth of the market is attributed to the rising incidence of chromosomal abnormalities associated with advanced maternal age.
Key Market Trends & Insights
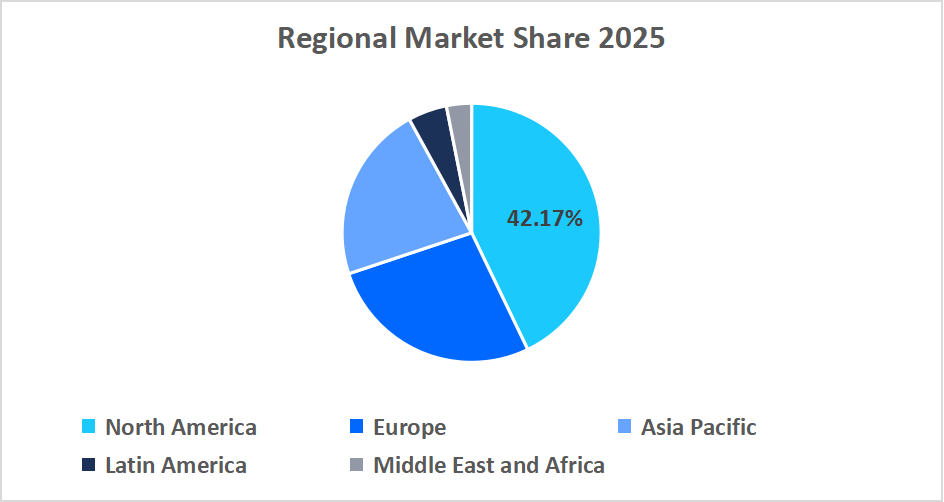
- North America held a dominant share of the global market, accounting for 42.17% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 41%.
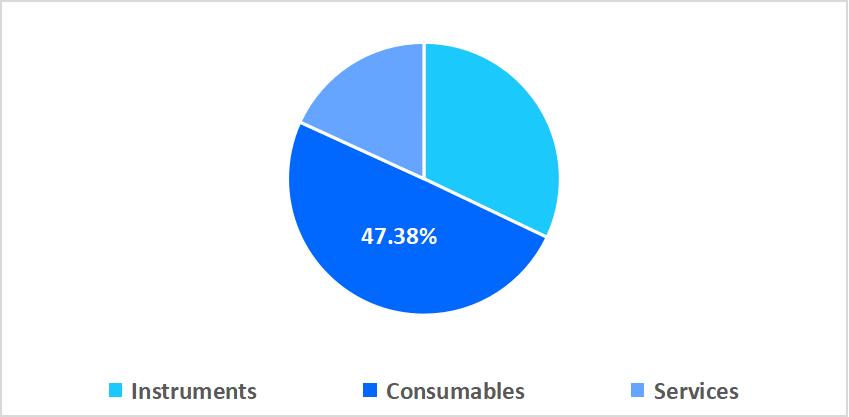
- Based on product, the consumables segment held the highest market share of 47.38% in 2025.
- By application, the microdeletion syndrome segment is expected to register the fastest CAGR growth of 96%.
- Based on the end user, the hospital segment dominated the market in 2025.
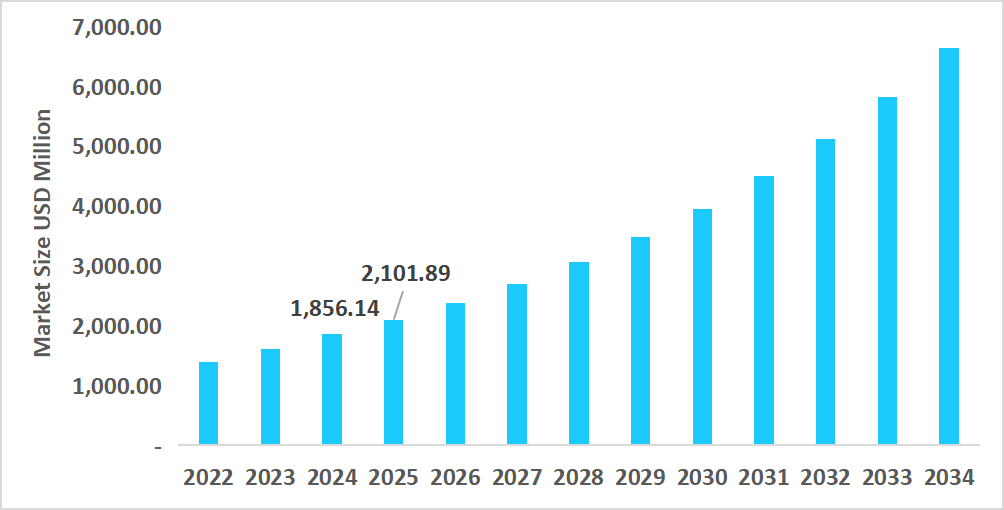
- The U.S. dominates the Non-invasive Prenatal Testing market, valued at USD 1.85 billion in 2024 and reaching USD 2.10 billion in 2025.
Table: U.S. Non-invasive Prenatal Testing Market Size (USD Million)
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 5.37 billion
- 2034 Projected Market Size: USD 16.94 billion
- CAGR (2026-2034): 13,67%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The global non-invasive prenatal testing (NIPT) market encompasses a wide range of diagnostic solutions. These solutions include advanced instruments such as ultrasound devices, NGS platforms, PCR instruments, microarray, and other instruments, supported by essential consumables such as assay kits, reagents, and disposables. In addition to products, the market provides comprehensive services for prenatal care. NIPT is applied across various clinical indications, including trisomy, microdeletion syndromes, and others. Moreover, these tests are provided through hospitals, clinics, and diagnostic laboratories.
Latest Market Trends
Growing use of Next-Generation Sequencing Tests
Prenatal genetic testing is shifting from limited trisomy screening toward comprehensive full genome analysis enabled by next generation sequencing (NGS) technologies. Traditionally, prenatal screening focused primarily on detecting chromosomal abnormalities such as Down syndrome, offering limited insights into broader genetic risks. With the advent of advanced sequencing platforms, this landscape is rapidly evolving. Roche’s introduction of its breakthrough Sequencing by Expansion (SBX) technology represents a new category in NGS, enabling ultra high accuracy and resolution in genome diagnostics.
These innovations are redefining prenatal care by providing detailed genomic insights that enhance pregnancy management and expand the clinical utility of genetic testing.
Shift from Invasive Diagnostic Procedures to Non-Invasive Prenatal Screening
The shift from traditional invasive diagnostic procedures, such as amniocentesis and chorionic villus sampling, to non-invasive prenatal testing is a major trend boosting market growth. The next-generation sequencing enables highly accurate detection of chromosomal abnormalities through a simple maternal blood draw, which reduces the risk of miscarriage associated with invasive methods
Therefore, growing preference of healthcare providers and patients for non-invasive, accurate, and safer prenatal testing signals a major trend in prenatal care practices.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 5.37 Billion |
| Estimated 2026 Value | USD 6.08 Billion |
| Projected 2034 Value | USD 16.94 Billion |
| CAGR (2026-2034) | 13.67% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Illumina, Inc., Natera, Inc., Laboratory Corporation of America Holdings, Eurofins Scientific, Hoffmann-La Roche AG |

to learn more about this report Download Free Sample Report
Non-invasive Prenatal Testing Market Drivers
Improvements in Reimbursement Policies for Average and Low-Risk Pregnancies
The expansion of reimbursement policies to include non-invasive prenatal testing for average and low risk pregnancies is a substantial driver of market growth. For example, as of July 1, 2024, Texas Medicaid removed the prior authorization requirement for NIPT for all pregnant individuals with a confirmed intrauterine singleton pregnancy dated at least ten weeks of gestation, regardless of risk status.
This policy change increased the adoption of NIPT testing, which, in turn, contributed to market growth.
Market Restraint
High Cost of NIPT Limits Market Adoption
A major restraint in the prenatal testing market is the high cost of non-invasive prenatal testing, which limits patient access and adoption. According to the American College of Obstetricians and Gynecologists (ACOG) 2023 report, the average out-of-pocket cost for NIPT in the U.S. ranges between USD 800 and 2,000 per test. Consequently, in regions with limited insurance reimbursement or coverage, this substantial expense makes NIPT unaffordable for a large portion of expectant parents. As a result, the high price of NIPT continues to act as a key barrier to its widespread utilization and market penetration globally.
Market Opportunity
Growing Use of Non-Invasive Prenatal Testing in Emerging Markets
The growing healthcare infrastructure and rising awareness in emerging markets are creating new growth opportunities in the non-invasive prenatal testing market. For example, in 2024, Illumina partnered with local diagnostic providers in India and launched its VeriSeq NIPT Solution, enabling access to prenatal screening for a larger population at earlier gestational ages.
Therefore, improving accessibility and affordability supports the adoption of non-invasive prenatal testing and creates new market opportunities.
Regional Analysis
North America dominated the non-invasive prenatal testing market in 2025, accounting for 42.17% market share. This dominance is attributed to the strong presence of accredited genetic testing laboratories with advanced sequencing infrastructure and the rapid integration of AI-driven data analytics for precise prenatal risk assessment. Moreover, the widespread physician awareness and patient education programs support early adoption of advanced prenatal screening solutions.
The U.S. non-invasive prenatal testing market is boosted by the increasing adoption of NIPT for fetal aneuploidies. This shift is primarily due to NIPT's higher accuracy and safety compared to traditional prenatal screening methods. A study published in the American Journal of Obstetrics & Gynecology involving over 30,000 women supports the use of NIPT as a first line prenatal screening test for aneuploidy. The ability to detect fetal sex more accurately and identify vanishing twin pregnancies further increases the adoption of NIPT tests, leading to increased adoption rates.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 15.41% from 2026-2034, owing to the rapid expansion of regional genomic research collaborations that promote localized assay development and the growing government-backed prenatal screening initiatives aimed at reducing birth defect rates. Additionally, increasing investment in maternal health digitization enhances accessibility to advanced NIPT solutions across developing economies.
In China, the NIPT market is experiencing notable growth, primarily driven by the adoption of NIPT as a free program for low-risk pregnant women in Wuhan since 2019. A study conducted at the Wuhan Maternal and Child Healthcare Hospital analyzed data from 81,389 pregnant women, including 60,193 low-risk participants, to evaluate the performance and pregnancy outcomes of NIPT. This large-scale implementation has expanded access to NIPT in China.
Europe Market Insights
Europe’s non-invasive prenatal testing market is witnessing strong growth, driven by the implementation of region-wide genomic data integration, supporting personalized prenatal care and the rising emphasis on ethical genomics regulations ensuring standardized testing quality. Moreover, growing collaborations among European diagnostic firms accelerate technology adoption and expand access to advanced NIPT services.
In Germany, the NIPT market is experiencing notable growth, primarily due to its inclusion in the statutory health insurance benefits. The Federal Joint Committee authorized NIPT coverage under specific conditions, such as abnormal ultrasound findings or after intensive counseling by qualified specialists. This policy ensures that NIPT is accessible to pregnant women who meet the criteria, thereby contributing to the expansion of the non-invasive prenatal testing market size in Germany.
Latin America Market Insights
Latin America’s non-invasive prenatal testing market is growing rapidly due to the establishment of regional genomic sequencing hubs that lower testing costs and improve sample logistics. Additionally, the increasing involvement of private companies in expanding prenatal screening awareness and training healthcare professionals is enhancing early diagnosis capabilities, driving broader adoption of advanced NIPT solutions across the region.
Argentina’s non-invasive prenatal testing market growth is supported by the government’s strategic integration of NIPT within national maternal fetal health programs, through public hospital collaborations that subsidize testing for high-risk pregnancies. This initiative increases accessibility for low-income populations and strengthens early genetic screening adoption nationwide.
Middle East and Africa Market Insights
The Middle East and Africa region is experiencing consistent NIPT market growth driven by the rising demand for prenatal genetic screening among populations with high consanguinity rates, which increases genetic disorder risks and fuels early screening adoption. Additionally, the international biotech companies are introducing economic NIPT assays tailored to across the region.
Egypt’s non-invasive prenatal testing market is expanding due to the introduction of localized genomic reference databases developed through collaborations between national research institutes and healthcare universities. These databases enhance the accuracy of NIPT result interpretation for the Egyptian population, promoting greater clinical reliability and encouraging adoption among obstetric specialists.
Regional Market share (%) in 2025
Source: Straits Research
Product Insights
The consumables segment dominated the market with a revenue share of 47.38% in 2025. This growth is supported by the rising demand for single-use sample preparation kits and reagents that ensure contamination-free testing. These consumables enhance testing accuracy, streamline workflow efficiency, and support higher throughput in prenatal laboratories, and drive segment expansion.
The services segment is projected to register the fastest CAGR of 14.31% during the forecast period, due to the increasing outsourcing of genomic data interpretation and bioinformatics analysis to specialized service providers, enabling laboratories to reduce operational burdens, improve turnaround times, and deliver clinically actionable prenatal insights more efficiently.
By Product Market Share (%), 2025
Source: Straits Research
Application Insights
The trisomy segment dominated the market with 49.41% in 2025, owing to the integration of advanced bioinformatics algorithms that enhance the detection sensitivity for chromosomal aneuploidies such as trisomy 21, 18, and 13. These algorithms minimize false-positive results, improving clinical confidence and driving wider adoption of trisomy-focused prenatal screening.
The microdeletion syndrome segment is expected to register the fastest CAGR growth of 14.96% during the forecast period due to the rising clinical implementation of high-resolution genomic assays capable of detecting smaller chromosomal deletions, enabling early identification of rare genetic disorders and improving prenatal counseling accuracy for high-risk pregnancies.
End User Insights
The hospitals segment dominated the market in 2025. This growth is supported by the integration of multidisciplinary prenatal care units that combine obstetrics, genetics, and fetal medicine under one setting. This coordinated care model enhances test accessibility, ensures rapid result interpretation, and promotes comprehensive pregnancy management, driving segment growth.
The diagnostic laboratories segment is forecasted to grow at a CAGR of 15.07% during the forecast period, owing to the adoption of automated high-throughput sequencing systems that enable large-scale sample processing, reduce turnaround times, and enhance testing accuracy, supporting efficient delivery of non-invasive prenatal diagnostics.
Competitive Landscape
The global non-invasive prenatal testing market is semi-consolidated, with a few companies dominating the revenue share. The launches of new NIPT tests and genomic assays in recent years intensified the competition. Key global players in the market are Illumina, Natera, Thermo Fisher Scientific, LabCorp, Quest Diagnostics, Roche, BGI Genomics, and others.
These market players are focusing on strategic initiatives such as product approvals, collaborations, funding activities, mergers, and acquisitions to strengthen their competitive position and expand their market share.
MedGenome: An emerging market player
MedGenome Labs Ltd. is an emerging player in the non-invasive prenatal testing market and is a leading clinical genomics company based in India. The company specializes in providing advanced NIPT tests to address the growing demand for accurate and non-invasive prenatal screening methods. MedGenome's NIPT offerings are designed to detect chromosomal abnormalities such as Down syndrome, Edwards syndrome, and Patau syndrome with high accuracy, catering to the needs of expectant mothers seeking reliable prenatal care.
List of Key and Emerging Players in Non-invasive Prenatal Testing Market
- Illumina, Inc.
- Natera, Inc.
- Laboratory Corporation of America Holdings
- Eurofins Scientific
- Hoffmann-La Roche AG
- Revvity, Inc.
- Thermo Fisher Scientific Inc.
- Agilent Technologies
- GE HealthCare Technologies Inc.
- Quest Diagnostics Incorporated
- Sonic Healthcare Limited
- CENTOGENE N.V.
- QIAGEN N.V.
- PathWest Laboratory Medicine WA
- Myriad Genetics
- BGI Genomics Co., Ltd.
- Invitae Corporation
- MedGenome
- Cordlife
- Others
Strategic Initiatives
- August 2025: Natera, Inc., launched the Fetal Focus, a non-invasive prenatal test for inherited conditions.
- February 2025: Yourgene Health launched the IONA Care+, a non-invasive prenatal screening service for genetic conditions
- June 2024: Illumina Inc., a leader in biotechnology, launched DRAGEN v4.3. a latest version of the DRAGEN software for the analysis of next-generation sequencing data
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 5.37 Billion |
| Market Size in 2026 | USD 6.08 Billion |
| Market Size in 2034 | USD 16.94 Billion |
| CAGR | 13.67% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Non-invasive Prenatal Testing Market Segments
By Product
-
Instrument
- Ultrasound Devices
- NGS Systems
- PCR Instruments
- Microarray
- Others
-
Consumables
- Assay Kits & Reagents
- Disposables
- Services
By Application
- Trisomy
- Microdeletion Syndrome
- Others
By End User
- Hospitals
- Clinics
- Diagnostic Laboratories
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































