The global market for oncology biosimilars is estimated at USD 8 billion in 2025 and is projected to grow from USD 9.48 billion in 2026 to USD 36.90 billion in 2034, exhibiting a CAGR of 18.5% during the forecast period (2025–2034).
Oncology Biosimilar Market Size, Share & Trends Analysis Report By Product Type (Monoclonal Antibodies (mAbs), Hematopoietic Agents, Granulocyte Colony-Stimulating Factor (G-CSF), Immunomodulators), By Cancer Type (Breast Cancer, Lung Cancer, Colorectal Cancer, Cervical Cancer, Blood Cancer / Hematological Cancers, Other), By Route of Administration (Intravenous (IV), Subcutaneous (SC), Others), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Oncology Biosimilar Market Overview
The global oncology biosimilar market size was estimated at USD 8.00 billion in 2025, and is anticipated to grow from USD 9.48 billion in 2026 till USD 36.90 billion in 2034, growing at a CAGR of 18.5% from 2026-2034.
Key Market Trends & Insights
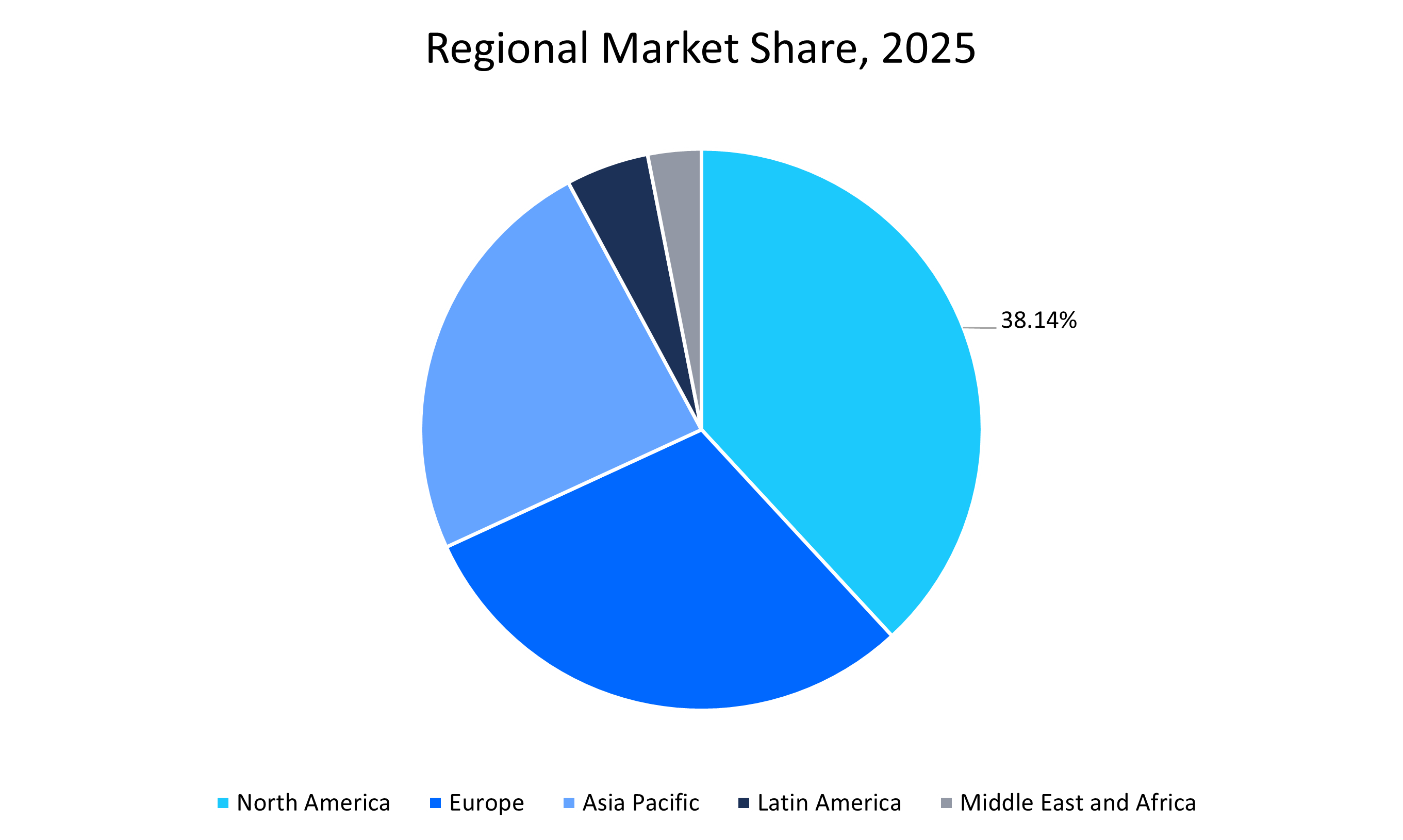
- North America dominated the market with a revenue share of 38.14% in 2025, attributed to robust healthcare infrastructure and supportive regulatory frameworks.
- Asia Pacific is emerging as the fastest-growing region in oncology biosimilars, exhibiting a CAGR of 19.8% in 2025.
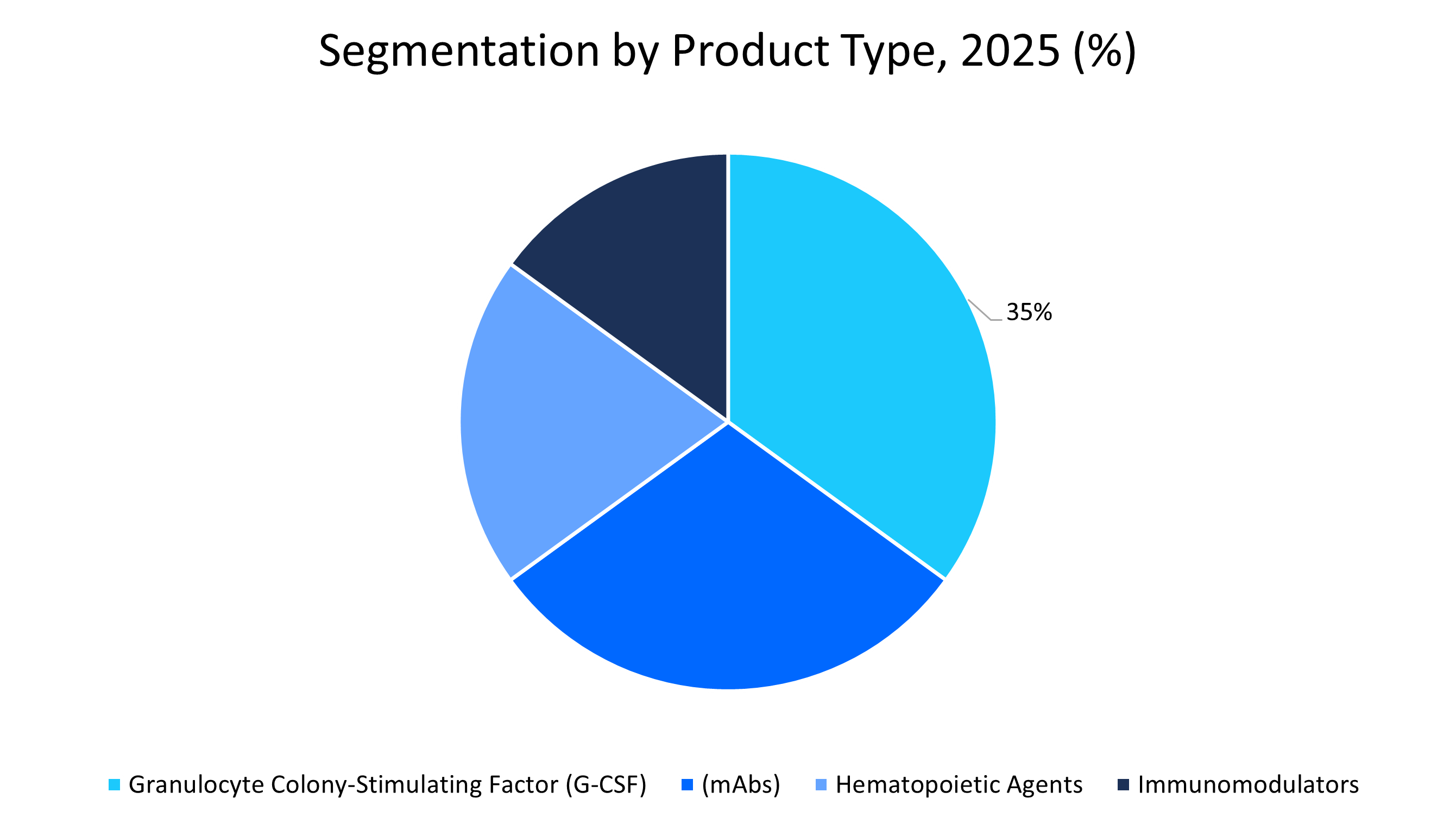
- By Product Type, Granulocyte Colony-Stimulating Factor (G-CSF) biosimilars held a revenue share of 35% in 2025.
- By Distribution Channel, Hospital pharmacies are estimated to grow at a CAGR of 18.2%.
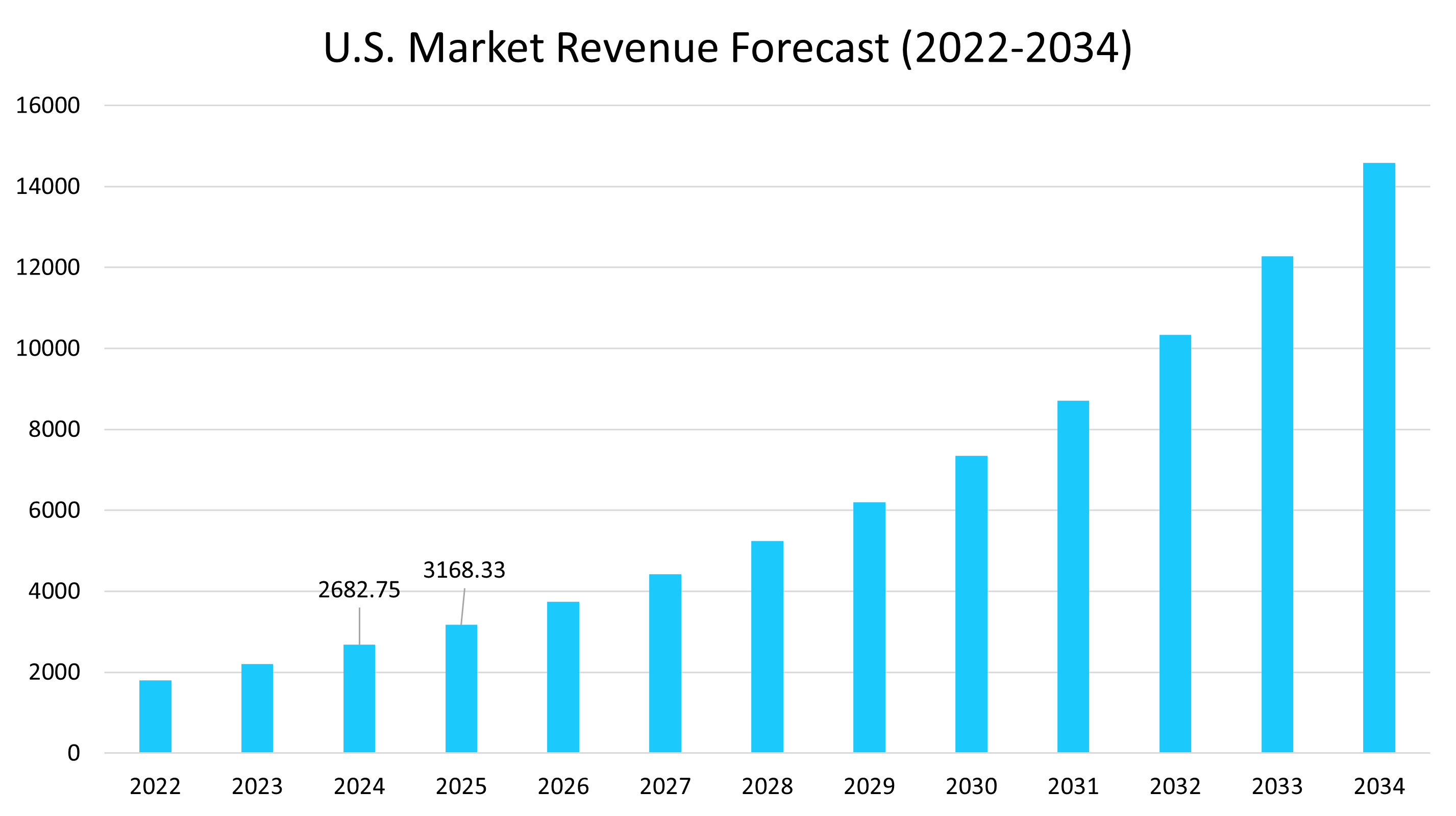
- The U.S. dominates the oncology biosimilar market, valued at USD 2.68 billion in 2024 and reaching USD 3.16 billion in 2025.
Market Size and Forecast
- 2025 Market Size: USD 8.00 billion
- 2034 Projected Market Size: USD 36.90 billion
- CAGR (2026–2034):5%
- Dominating region: North America
- Fastest-growing region: Asia Pacific
Source: Straits Research Analysis
The global oncology biosimilar market growth is attributed to patent expirations on biologic cancer therapies and robust regulatory approvals enabling lower-cost biosimilars to enhance patient access and reduce financial pressure on healthcare systems.
Oncology biosimilars are transforming cancer care by making advanced treatments both affordable and innovative. With supportive frameworks like the U.S. Biologics Price Competition and Innovation Act, these therapies benefit from faster approvals and reduced development costs. According to a PMC study, broader biosimilar adoption in the U.S. could generate USD 12–54 billion in savings by 2026. As cancer incidence continues to rise worldwide, healthcare payers are increasingly turning to biosimilars, such as trastuzumab and rituximab equivalents, to lower treatment costs without compromising on proven efficacy and safety.
Market Trends
Interchangeability and Clinical Education Driving Trust in Biosimilars
A major transformative development for the oncology biosimilars market is the FDA’s interchangeability designation, which allows pharmacists to substitute biosimilars for reference biologics without physician approval, streamlining access much like generic drugs. This regulatory step, combined with growing clinical education efforts, is strengthening trust among providers and patients, which is critical in oncology, where treatment decisions carry high stakes.
- According to a GaBI Journal report from May 2025, 73% of surveyed healthcare providers now express positive or very positive views of biosimilars, citing cost savings, safety, and efficacy as primary benefits.
This trend towards widespread education and regulatory harmonization is crucial for accelerating uptake and ensuring that the full potential of biosimilars is realized in delivering accessible and effective cancer care.
Hospital Pharmacies and Online Platforms Are Reshaping Distribution
The oncology biosimilar market is experiencing a structural shift in distribution, with hospital procurement and online pharmacy channels playing a pivotal role. Hospitals continue to serve as the primary hubs for biosimilar adoption due to their centralized procurement models and ability to handle complex oncology care.
At the same time, the rise of digital platforms is democratizing access, especially in underserved regions where patients often face delays in treatment due to tender-driven purchasing systems. The combined effect is wider market penetration, reduced treatment costs, and improved patient access, driving both equity and efficiency in oncology care.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 8.00 billion |
| Estimated 2026 Value | USD 9.48 billion |
| Projected 2034 Value | USD 36.90 billion |
| CAGR (2026-2034) | 18.5% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Pfizer Inc., Amgen Inc., Novartis International AG, Biocon Ltd., Reddy’s Laboratories |

to learn more about this report Download Free Sample Report
Market Drivers
Cost Savings Are Driving Widespread Adoption
Oncology biosimilar adoption offers an undeniable cost advantage. In a study mentioned in the National Library of Medicine, biosimilars achieved 56.4% uptake across Medicare-covered biologics in 2024, including bevacizumab, trastuzumab, rituximab, pegfilgrastim, and filgrastim, resulting in a 23% reduction in Medicare drug spending for those agents.
- According to the Center for Biosimilars, in inpatient settings between 2019 and 2024, biosimilars captured 57.9% utilization, with average wholesale acquisition cost reductions of 35% versus originators.
These savings allow healthcare systems to reallocate resources toward other oncology innovations, expanding patient access to life-saving biologics, and driving the market.
Regulatory Approvals Fueling Competition and Adoption
A rapidly evolving regulatory environment is reinforcing competition and accelerating adoption. Regulators worldwide are recognizing biosimilars as critical tools to lower cancer care costs, paving the way for wider adoption and stronger confidence among payers and providers. By the end of 2024, the FDA had approved 63 biosimilars, signaling consistent momentum in regulatory approvals.
- For example, in Q1 2025, Samsung Bioepis reported that biosimilar launches reached a 53% market share, leading to a 53% drop in average drug costs within just five years of market entry.
The synergy between regulatory confidence and competitive economics is accelerating oncology biosimilar adoption, ensuring both scale and sustainability in the market.
Market Restraint
Patent Litigation and Market Exclusivity Barriers
The entry of oncology biosimilars is often delayed by complex patent landscapes and prolonged litigation initiated by originator companies. Secondary patents covering formulation methods, delivery devices, or manufacturing processes extend exclusivity periods beyond the initial patent expiry.
In addition, data exclusivity provisions in several regions restrict biosimilar developers from leveraging reference clinical trial data for many years. In many regions, reference product data from clinical trials is legally protected for a fixed period (e.g., 8–12 years in the EU, 12 years in the US). These legal and regulatory hurdles stall timely biosimilar launches, reducing competitive intensity in the oncology space and limiting patient access to lower-cost alternatives.
Market Opportunity
Strategic Partnerships and Portfolio Expansion
Strategic alliances are proving to be powerful catalysts for oncology biosimilar growth. Companies are increasingly turning to partnerships, licensing agreements, and co-development deals to accelerate entry into high-value therapeutic areas, while regulators are widening the scope of approved oncology biosimilars, particularly in supportive care.
- For example, in June 2025, Dr. Reddy’s Laboratories partnered with Alvotech to co-develop a Keytruda (pembrolizumab) biosimilar, targeting one of the most lucrative oncology markets.
- In February 2025, the FDA approved multiple denosumab biosimilars (Ospomyv, Xbryk, Bomyntra, and Conexxence), expanding access in supportive oncology care, enhancing treatment diversity, and reinforcing the biosimilar value proposition for payers and providers.
By combining partnership strategies with diversified product portfolios, manufacturers are well-positioned to tap into various oncology segments, improve treatment accessibility, and sustain long-term market momentum.
Regional Analysis
North America Market Trends
North America region dominated the market with a revenue share of 38.14% in 2025. This growth is attributed to its robust healthcare infrastructure and supportive regulatory frameworks. The region’s established reimbursement systems, biosimilar substitution policies, and efficient approval pathways encourage institutional adoption and cost savings. Government-led initiatives such as the Biologics Price Competition and Innovation Act have paved the way for biosimilar entry and competition in the U.S. over the last two decades. Major regional players such as Amgen, Pfizer, Samsung Bioepis, Dr. Reddy’s, Mylan, and Biocon are actively commercializing oncology biosimilars, strengthening market growth through competitive pricing and expanded access.
 Source: Straits Research
Source: Straits Research
Asia Pacific Market Growth Factors
Asia Pacific is emerging as the fastest-growing region in oncology biosimilars, exhibiting a CAGR of 19.8% in 2025. The rapid incidence of cancer and the expansion of local manufacturing, especially in China, India, and South Korea, have created a strong demand for affordable biosimilar options. National governments are strengthening regulatory standards to boost both safety and global competitiveness. Regional firms are forging critical collaborations and commercialization avenues, fueling market penetration amid rising patient access and cost pressures. This dynamic environment positions the Asia Pacific as a key market frontier for oncology biosimilars.
Europe Market Trends
Europe holds a strong position due to favorable reimbursement systems, substitution policies, and mature regulatory mechanisms that facilitate uptake. The availability of multiple mAb biosimilars, including five rituximab, six trastuzumab, and eight bevacizumab biosimilars, reflects both market maturity and physician confidence. Additionally, the European Commission approved an aflibercept biosimilar (Ahzantive/Baiama) in January 2025 through a partnership involving Formycon and Teva. Governments across Europe also embed biosimilar usage in cost containment and access frameworks, enabling insurers and health systems to reinvest savings into innovation and broader oncology therapies.
Countries Analysis
U.s. Market Trends
The U.S. dominates the oncology biosimilar market, benefiting from the Biologics Price Competition and Innovation Act (BPCIA, 2010), which created a clear approval pathway. According to the Association for Accessible Medicines, biosimilars generated $12.6 billion in savings in 2023 alone, contributing to over USD 445 billion in combined savings from generics and biosimilars. The FDA approved 63 biosimilars in 2024, a record high, signaling accelerating regulatory momentum. Hospitals and large insurers increasingly favor biosimilars due to their 30–80% cost discount compared to originators. With cancer spending surpassing $70 billion annually, the U.S. represents a cornerstone growth hub for oncology biosimilars.
Germany Market Growth Factors
Germany is one of Europe’s strongest adopters of oncology biosimilars, owing to robust reimbursement policies and the AMNOG framework for cost-effectiveness assessments. German statutory health insurance providers actively encourage biosimilar substitution, with some regions reporting uptake rates above 80% for trastuzumab and rituximab biosimilars by 2024. With over 500,000 new cancer cases diagnosed annually (Robert Koch Institute, 2024), Germany’s market remains critical in Europe.
Uk Market Trends
The UK’s National Health Service (NHS) has been a pioneer in promoting biosimilars through centralized tenders and cost-saving mandates. NHS England estimates that biosimilars generate over £300 million in annual savings, much of which is reinvested in innovative treatments such as CAR-T therapies. This positions the UK as a global model for systematic biosimilar integration in healthcare, showcasing how robust policy frameworks can translate into sustainable patient access and reinvestment in cutting-edge therapies.
China Market Growth Factors
China’s oncology biosimilar market is expanding rapidly amid a growing cancer burden, with an estimated 3,246,625 new cancer cases reported in 2024 as per the National Library of Medicine. Government policies like the Volume-Based Procurement (VBP) program accelerate biosimilar adoption by cutting drug prices. Domestic players such as Henlius, Innovent Biologics, and Bio-Thera Solutions lead the local biosimilar pipeline. Rituximab and trastuzumab biosimilars are already available, and expanding oncology biosimilar adoption is expected to save billions for China’s public health system in the coming decade.
India Market Trends
India represents a fast-emerging hub for oncology biosimilars, driven by high cancer incidence, with an incidence of 708,223 cases reported in the National Cancer Registry Programme Investigator Group Report in 2025, and the country’s role as a low-cost global manufacturing base. Companies such as Biocon, Dr. Reddy’s, Intas, and Zydus Cadila dominate the domestic biosimilar landscape, with exports to the U.S., EU, and emerging markets. Biocon’s trastuzumab biosimilar (Ogivri) became the first FDA-approved biosimilar from India, setting a precedent for global entry. India’s cost advantage positions it as both a domestic supplier and an international partner in oncology biosimilars.
Market Segmentation
Product Type Insights
Granulocyte Colony-Stimulating Factor (G-CSF) biosimilars segment dominated the market with a revenue share of 35% in 2025. The growth is attributed to their critical role in oncology care for mitigating neutropenia during chemotherapy. Notable FDA-approved G-CSF biosimilars, such as Zarxio, Zefylti, and Nivestym, have become widely adopted owing to cost-effectiveness and clinical familiarity among oncologists, particularly in supportive care protocols. Moreover, the segment is supported by high adoption in both inpatient and outpatient chemotherapy settings.
Source: Straits Research
Cancer Type Insights
Breast cancer biosimilars dominated the market in 2025 and is expected to grow at a CAGR of 17.8%. This leadership stems from the high global incidence of breast cancer, with over 310.720 new invasive breast cancer cases estimated to be diagnosed among women in 2024 by American Cancer Society. Trastuzumab-based biosimilars such as Kanjinti (Amgen/Allergan), Trazimera (Pfizer), Herzuma (Celltrion/Teva), Ontruzant (Samsung Bioepis), and Ogivri (Biocon/Viatris) have driven this growth, as HER2-positive breast cancer remains a leading indication for biopharmaceuticals.
Route of Administration Insights
The intravenous (IV) segment stands out as both dominant and fastest growing too. Oncology treatment continues to rely heavily on hospital-based infusion protocols, where IV delivery ensures accurate dosing, aseptic administration, and integration with complex regimens. IV remains the gold standard for mAbs (trastuzumab, bevacizumab, rituximab) as well as hematopoietic agents, making it foundational to current treatment delivery models. According to NIM, a majority of oncology biosimilars in use today are IV formulations, and hospital infusion centers remain critical in driving biosimilar uptake.
Distribution Channel Insights
Hospital pharmacies segment dominated the market with a revenue share of 42% in 2025. Hospitals are the primary venues for administering complex biologic therapies, offering formulary control, clinical oversight, proper cold chain storage infrastructure, and patient education, all essential for biosimilar deployment. As per NIH, in the U.S., hospitals and clinics accounted for over 40% of oncology biosimilar sales, while Europe’s tender-driven hospital systems reinforced this dominance.
Competitive Landscape
The global oncology biosimilar market is moderately fragmented, shaped by many players ranging from large pharmaceutical firms to emerging biosimilar specialists. This fragmentation fosters competitive pricing, broad access, and regional portfolio specialization, while large-cap companies anchor the market through global scale and deep regulatory expertise.
The industry participants are inclined towards high R&D and manufacturing investment required to develop oncology biosimilars, prompting strategic alliances and licensing rather than monopolistic dominance.
Dr. Reddy’s Laboratories: An Emerging Player in the Market
Dr. Reddy’s Laboratories has steadily expanded its oncology biosimilar footprint through strategic partnerships and the development of high-value monoclonal antibody alternatives. Its collaboration with global firms positions it as a cost-effective manufacturer with a growing global reach.
- In June 2025, Dr. Reddy’s announced a collaboration with Alvotech to co-develop, manufacture, and commercialize a Keytruda (pembrolizumab) biosimilar, leveraging Alvotech’s biotech expertise and Dr. Reddy’s commercialization capabilities to access high-impact immunotherapy markets.
List of Key and Emerging Players in Oncology Biosimilar Market
- Pfizer Inc.
- Amgen Inc.
- Novartis International AG
- Biocon Ltd.
- Reddy’s Laboratories
- Samsung Bioepis
- Mylan N.V. (now part of Viatris)
- Allergan (AbbVie)
- STADA Arzneimittel AG
- Apotex Inc.
- Roche (via biosimilar affiliates)
- Sandoz (Novartis division)
- Celltrion Inc.
- Bio-Thera Solutions
- Organon (via acquired biosimilar rights)
- Teva Pharmaceuticals
to learn more about this report Download Market Share
Recent Developments
- March 2025- In Q1 2025, the FDA approved six denosumab biosimilars (supportive care agents crucial in oncology for bone-related complications), reinforcing uptake in cancer supportive regimens.
- April 2025-Samsung Bioepis and Teva launched Epysqli in the U.S., an eculizumab biosimilar, offered at a 30% discount compared to the reference product, Soliris, expanding affordability and access for patients with complement-mediated disorders often relevant in oncology settings.
Analyst Opinion
As per our analyst, the global oncology biosimilar market expansion is fueled by a confluence of factors, patent expirations of blockbuster biologics, streamlined regulatory pathways, and stronger payer support for cost-saving alternatives. This shift is already visible, with biosimilars like bevacizumab capturing up to 90% of market share by Q3 2024. Approvals of supportive care biosimilars such as denosumab and eculizumab are further widening the treatment landscape, while high-profile alliances, like Keytruda biosimilar partnerships, are unlocking entry into lucrative immunotherapy segments. For manufacturers, oncology biosimilars are becoming a necessity. Companies that embrace strategic collaborations and deliver both affordability and reliability will not only strengthen adoption and access but also cement biosimilars as a cornerstone of modern cancer care.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 8.00 billion |
| Market Size in 2026 | USD 9.48 billion |
| Market Size in 2034 | USD 36.90 billion |
| CAGR | 18.5% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Cancer Type, By Route of Administration, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Oncology Biosimilar Market Segments
By Product Type
- Monoclonal Antibodies (mAbs)
- Hematopoietic Agents
- Granulocyte Colony-Stimulating Factor (G-CSF)
- Immunomodulators
By Cancer Type
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Cervical Cancer
- Blood Cancer / Hematological Cancers
- Other types (e.g., kidney, stomach, brain cancers)
By Route of Administration
- Intravenous (IV)
- Subcutaneous (SC)
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































