Pet Dewormers Market Size, Share & Trends Analysis Report By Pet (Dogs, Cats, Horses, Other Pets), By Route of Administration (Oral, Injectable, Topical), By Dosage Form (Solid, Liquid, Others), By Type (OTC, Prescription), By Distribution Channel (Veterinary Hospitals/Clinics, Retail/Pet Stores, E-commerce) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Pet Dewormers Market Overview
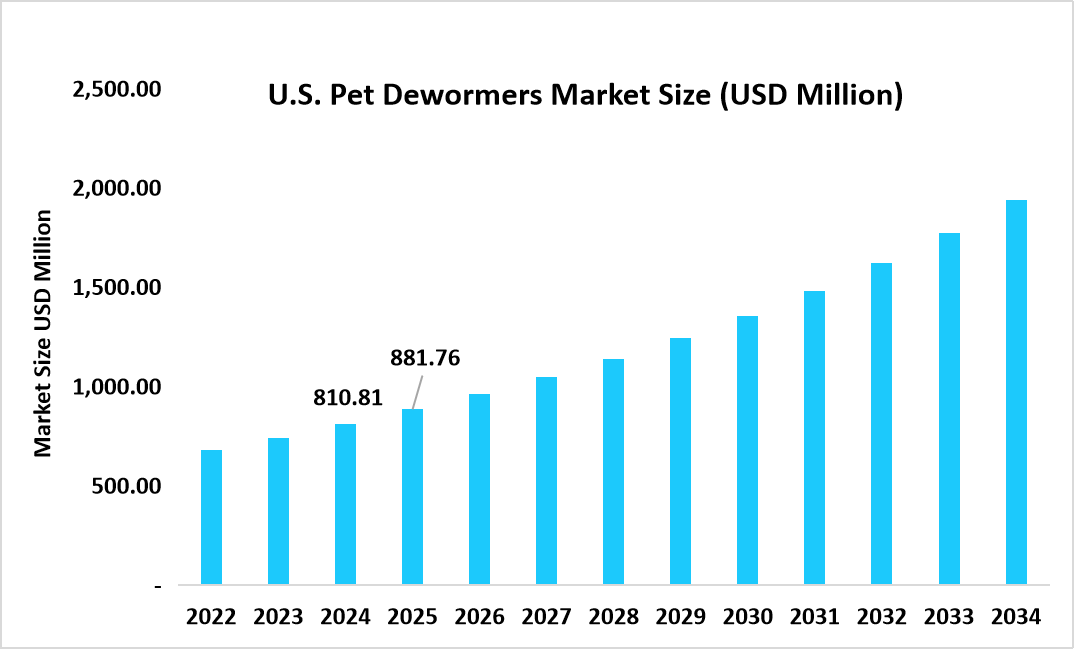
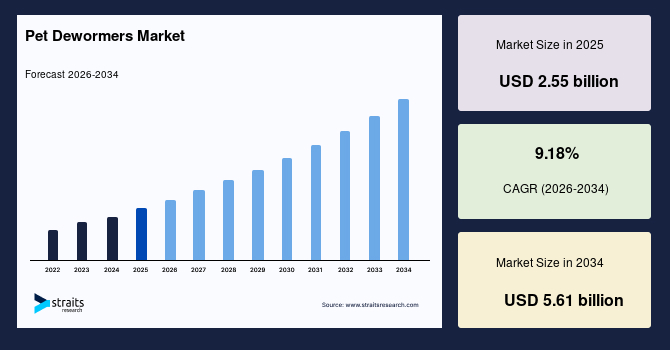
The global pet dewormers market size is valued at USD 2.55 billion in 2025 and is estimated to reach USD 5.61 billion by 2034, growing at a CAGR of 9.18% during the forecast period. The market growth is accelerated by the rising integration of routine parasite prevention into companion animal wellness plans supported by expanding veterinary prescription programs and regulatory-approved combination anthelmintic products.
Key Market Trends & Insights
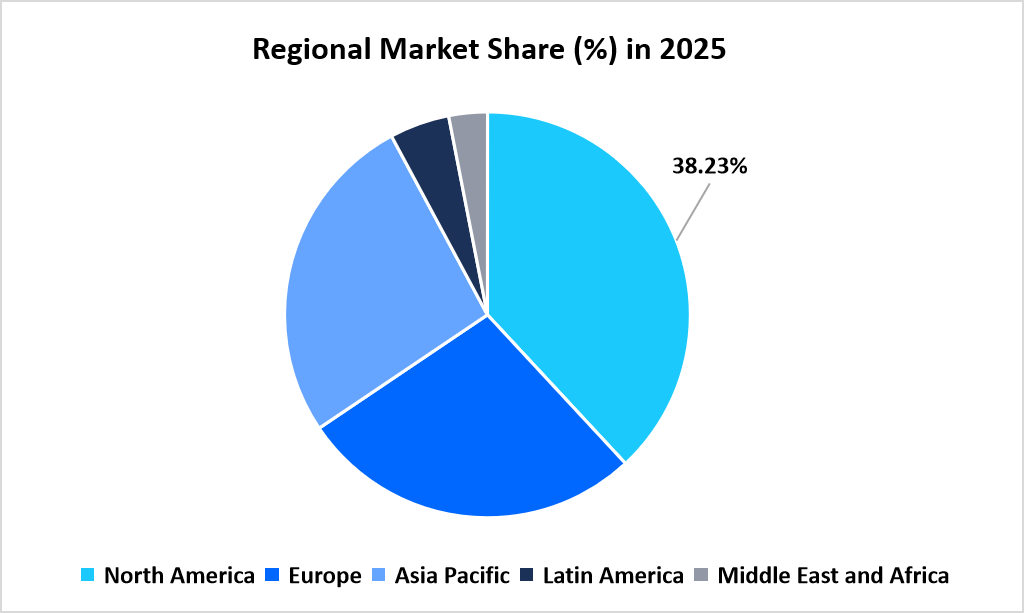
- North America held a dominant share of the global market, accounting for 38.23% share in 2025.
- The Asia Pacific region is forecasted to grow at the fastest pace, with a CAGR of 11.18%during the forecast timeframe.
- Based on Pet, dogs segment dominated the market with a revenue share of 46.66% in 2025.
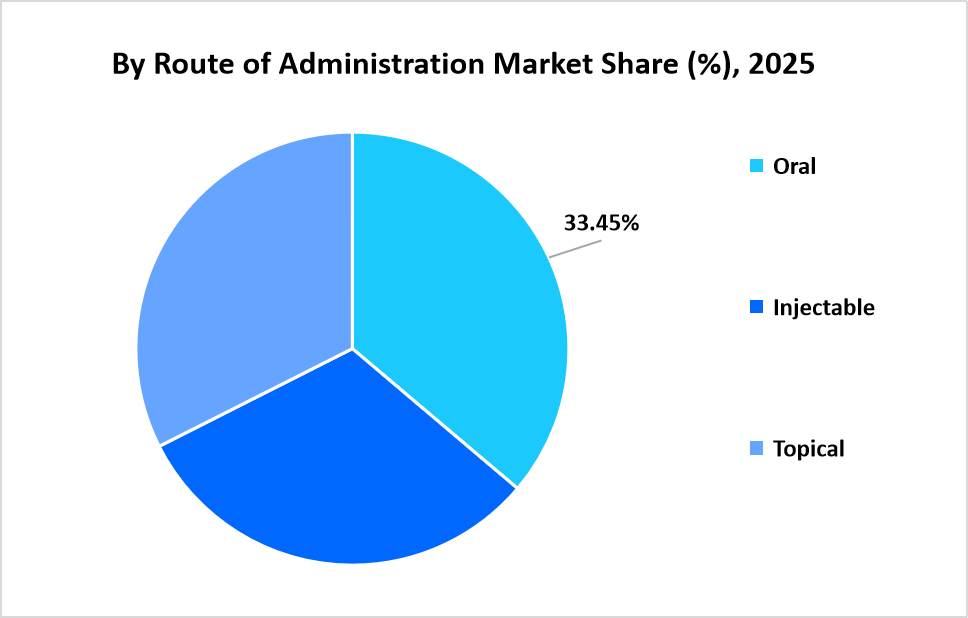
- Based on Route of Administration, oral segment dominated the market with a revenue share of 33.45% in 2025.
- Based on Dosage Form, solid segment dominated the market with a revenue share of 49.25% in 2025.
- Based on Type, OTC segment is anticipated to register the fastest CAGR of 10.88% during the forecast period.
- Based on Distribution Channel, veterinary hospitals/clinics segment dominated the market with a revenue share of 43.46% in 2025.
- The U.S. dominates the market, valued at USD 810.81 million in 2024 and reaching USD 881.76 million in 2025.
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.55 billion
- 2034 Projected Market Size: USD 5.61 billion
- CAGR (2026-2034): 9.18%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The pet dewormers market comprises veterinary pharmaceutical products formulated to prevent and treat internal parasitic infections in companion and leisure animals by eliminating gastrointestinal helminths such as roundworms hookworms tapeworms and whipworms. The market includes products administered to dogs cats horses and other pets through oral injectable and topical routes and is available in solid liquid and other dosage forms to suit species specific dosing and compliance requirements. Pet dewormers are distributed as over the counter and prescription products depending on active ingredients spectrum and regulatory classification and are supplied through veterinary hospitals and clinics retail and pet stores and e commerce platforms. Market activity is shaped by routine parasite control protocols regulatory approval standards and the integration of deworming into preventive veterinary care programs for companion animals.
Market Trends
Shift From Single-Molecule Dewormers to Combination-Spectrum Parasite Control
The pet dewormers market is witnessing a shift from single active ingredient products toward combination spectrum formulations that address multiple intestinal parasites within one dosing regimen. Regulatory labeling updates and veterinary prescribing patterns increasingly favor products combining agents such as praziquantel milbemycin or pyrantel to cover roundworms hookworms and tapeworms simultaneously. This shift is driven by rising awareness of mixed parasite burdens in companion animals and alignment with structured parasite prevention frameworks documented by government veterinary authorities. Combination products reduce treatment frequency and support standardized parasite management across companion animal populations.
Shift From Periodic Deworming Schedules to Preventive Lifecycle Parasite Management
The market is shifting from intermittent symptom-driven deworming toward lifecycle-based preventive parasite control embedded within routine wellness programs. Veterinary product labeling and parasite risk advisories published on official .gov and .org platforms increasingly emphasize age-specific and lifestyle-specific dosing intervals. This shift supports earlier initiation of deworming in puppies kittens and high exposure pets and reinforces recurring demand tied to preventive care rather than episodic treatment.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.55 billion |
| Estimated 2026 Value | USD 2.78 billion |
| Projected 2034 Value | USD 5.61 billion |
| CAGR (2026-2034) | 9.18% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Zoetis, Boehringer Ingelheim, Merck & Co., Inc., Elanco Animal Health, Dechra Pharmaceuticals PLC |
to learn more about this report Download Free Sample Report
Market Driver
Expansion of Companion Animal Parasite Prevention Portfolios By Leading Manufacturers
A key driver of the pet dewormers market is the expansion of companion animal parasite prevention portfolios by established animal health companies through formulation upgrades lifecycle extensions and geographic rollout. In 2024, Zoetis announced expanded availability and labeling updates for its companion animal parasiticide portfolio targeting broader intestinal parasite coverage across dogs and cats as disclosed in official company communications. These portfolio expansions align with regulatory approvals issued by the U.S. Food and Drug Administration Center for Veterinary Medicine and reinforce sustained prescription-driven demand through veterinary channels.
Market Restraint
Regulatory Complexity And Prolonged Approval Timelines For Veterinary Anthelmintics
The pet dewormers market faces restraint from multi-stage regulatory review processes governing safety efficacy residue exposure and environmental impact of veterinary anthelmintics. Regulatory authorities require extensive pharmacokinetic toxicology and post approval monitoring data before granting or extending companion animal indications. These requirements increase development timelines and compliance costs, particularly for combination products and new dosage formats, limiting rapid market entry and portfolio diversification.
Market Opportunity
Growth of Region-Specific Parasite Control Products Aligned With Climate And Lifestyle Exposure
An emerging opportunity lies in development of region-specific pet dewormers tailored to localized parasite prevalence climate conditions and pet lifestyle patterns. Government animal health agencies and academic institutions continue publishing region-based parasite surveillance data through official platforms which supports differentiated product positioning. Manufacturers integrating localized risk data into dosing guidance formulation strength and labeling strategy gain opportunities to expand adoption across urban and semi-urban pet populations while aligning with regulatory expectations for targeted parasite control.
Regional Analysis
North America led the pet dewormers market in 2025 with a share of 38.23%, supported by structured companion animal parasite control frameworks and high veterinary prescription adherence. Market development is reinforced by guidance issued by the Companion Animal Parasite Council, which publishes region-specific intestinal parasite risk maps and deworming frequency recommendations through its official .org platform.
In the U.S., market growth is supported by regulatory approvals and post approval product maintenance under the U.S. Food and Drug Administration Center for Veterinary Medicine. Product portfolio disclosures by Zoetis reflect expanded availability of combination oral dewormers for dogs and cats across veterinary clinics and hospital networks, aligned with prescription-based parasite prevention protocols.
Asia Pacific Pet Dewormers Market Insights
Asia Pacific is emerging as a fast-expanding region of 11.18% CAGR due to increasing formalization of companion animal healthcare and regulatory standardization. Regional market growth is supported by domestic manufacturing and government-issued animal health advisories addressing pet parasite management.
India’s pet dewormers industry is driven by regulatory oversight from the Department of Animal Husbandry and Dairying, which publishes companion animal parasite control guidance through official .gov portals. Portfolio expansion activities by Intas Pharmaceuticals Ltd. indicate increased availability of albendazole and praziquantel-based formulations supplied to urban veterinary clinics and licensed retail pharmacies.
Source: Straits Research
Europe Market Insights
Europe’s pet dewormers market growth is supported by centralized veterinary medicinal regulation and harmonized pharmacovigilance systems. Oversight by the European Medicines Agency governs approval renewals, labeling compliance and safety reporting for companion animal anthelmintics across member states.
Germany’s market development is augmented by parasite surveillance frameworks coordinated under federal veterinary public health authorities, which publish zoonotic helminth monitoring updates through official government channels. Strategic product positioning by Boehringer Ingelheim supports continued distribution of praziquantel and fenbendazole-based dewormers through regulated veterinary supply chains.
Latin America Market Insights
Latin America’s pet dewormers market growth is supported by public health-oriented parasite control initiatives linking animal and human health outcomes. Veterinary authorities across the region emphasize routine intestinal parasite management in companion animals within zoonotic disease prevention programs.
Brazil’s market expansion is guided by regulatory supervision from the Ministério da Agricultura e Pecuária, which oversees veterinary pharmaceutical registration and local manufacturing compliance. Regional supply expansion by Ceva Santé Animale supports increased distribution of liquid and chewable dewormers adapted to local veterinary prescribing practices.
Middle East and Africa Market Insights
The Middle East and Africa region is witnessing steady market development driven by rising companion animal ownership in urban centers and strengthening veterinary regulatory frameworks. Government animal health departments increasingly reference parasite control within companion animal welfare policies.
South Africa’s market for pet dewormers growth is shaped by regulatory standards issued by the Department of Agriculture Land Reform and Rural Development, which governs veterinary product registration and controlled distribution. Distribution expansion initiatives by Vetoquinol support access to tablet and suspension-based dewormers across Southern and Eastern African veterinary channels.
Pet Insights
The dogs segment dominated, accounting for 46.66% of the market in 2025. This dominance is driven by structured parasite control protocols embedded within veterinary drug labeling and approval conditions regulated by the U.S. Food and Drug Administration Center for Veterinary Medicine, which mandates routine intestinal parasite coverage for canine products with zoonotic risk claims.
The cats segment is the fastest growing, registering 10.21%, supported by increasing regulatory approvals of feline-specific anthelmintic formulations and expanded labeling for indoor and outdoor parasite exposure scenarios documented through official veterinary drug databases.
Route of Administration Insights
The oral segment dominated the market with 33.45%, supported by widespread regulatory approval of chewable tablets and flavoured oral suspensions listed within government-maintained veterinary medicinal product registries. Oral formulations remain the primary route for combination dewormers integrating roundworm, hookworm, and tapeworm coverage.
The topical segment is the fastest growing, accounting for 10.45%, driven by manufacturer introductions of spot-on parasiticides with intestinal parasite claims disclosed through veterinary product approval notifications and press communications.
Source: Straits Research
Dosage Form Insights
The solid dosage form segment dominated, holding 49.25% of the market in 2025, due to extended shelf stability, transport efficiency and standardized dosing referenced within approved product specifications published by regulatory authorities.
The other segment is the fastest growing, accounting for 10.67%, driven by increased availability of paste-based and combination delivery systems introduced through manufacturer product updates aimed at improved palatability.
Type Insights
The prescription segment dominated the market, supported by regulatory frameworks that restrict access to broad-spectrum and combination dewormers through licensed veterinary channels to manage resistance and zoonotic transmission risks.
The OTC segment is the fastest growing, registering 10.88%, driven by expanded approval of single active ingredient products distributed through licensed retail outlets under national veterinary drug regulations.
Distribution Channel Insights
The veterinary hospitals and clinics segment dominated, accounting for 43.46%, due to controlled dispensing of prescription dewormers aligned with diagnostic screening and treatment protocols regulated by veterinary medical authorities.
The e-commerce segment is the fastest growing, holding 10.91%, supported by government-authorized online veterinary pharmacies and manufacturer direct to consumer distribution models operating under pharmaceutical compliance requirements.
Competitive Landscape
The global pet dewormers market is moderately consolidated, with established animal health companies including Zoetis, Boehringer Ingelheim, Merck & Co., Inc., Elanco Animal Health, Dechra Pharmaceuticals PLC, Virbac, Vetoquinol, and Ceva Santé Animale accounting for a large share of commercial sales.
-
Hester Biosciences Limited: An emerging market player
Hester Biosciences Limited represents an emerging participant in the market with growing emphasis on companion animal therapeutics alongside its livestock portfolio. The company has expanded its veterinary pharmaceutical offerings through domestic manufacturing and distribution partnerships targeting urban veterinary clinics and retail channels. Product registrations, formulation diversification and regional market penetration initiatives position Hester Biosciences as a developing player addressing the rising demand for routine parasite control in pets.
List of Key and Emerging Players in Pet Dewormers Market
- Zoetis
- Boehringer Ingelheim
- Merck & Co., Inc.
- Elanco Animal Health
- Dechra Pharmaceuticals PLC
- Virbac
- Vetoquinol
- Hester Biosciences Limited
- Ceva Santé Animale
- Intas Pharmaceuticals Ltd.
- Norbrook Laboratories
- Phibro Animal Health Corporation
- Jurox Pty Ltd.
- Huvepharma
- Sogeval
- Others
Recent Developments
- November 2025: Elanco received the first FDA Emergency Use Authorization for Credelio CAT (lotilaner) to treat New World screwworm infestations in cats, preparing veterinarians and pet owners before the parasite reaches the U.S.
- September 2025: Elanco’s Credelio Quattro achieved blockbuster status, offering the broadest isoxazoline parasite protection against fleas, ticks, heartworm, and intestinal worms, marking a major advancement in comprehensive canine parasite control.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.55 billion |
| Market Size in 2026 | USD 2.78 billion |
| Market Size in 2034 | USD 5.61 billion |
| CAGR | 9.18% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Pet, By Route of Administration, By Dosage Form, By Type, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Pet Dewormers Market Segments
By Pet
- Dogs
- Cats
- Horses
- Other Pets
By Route of Administration
- Oral
- Injectable
- Topical
By Dosage Form
- Solid
- Liquid
- Others
By Type
- OTC
- Prescription
By Distribution Channel
- Veterinary Hospitals/Clinics
- Retail/Pet Stores
- E-commerce
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































