Point of Care Infectious Disease Testing Market Size, Share & Trends Analysis Report By Product (Consumables, Instruments, Software and Services), By Technology (Lateral Flow Immunoassay, Agglutination Test, Flow-through test/ Immunoconcentration Assay, Molecular Diagnostics, Others), By Application (Respiratory Infection Testing Products, Healthcare-Associated Infection Testing Products, Tropical Disease Testing Products, Sexually Transmitted Disease Testing Products), By End Use (Hospital, Diagnostic Laboratories, Home-care settings, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Point of Care Infectious Disease Testing Market Overview
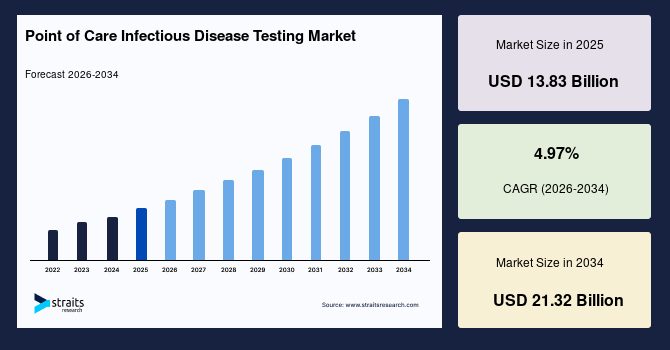
The global point of care infectious disease testing market size is estimated at USD 13.83 billion in 2025, and is projected to reach USD 21.32 billion by 2034, growing at a CAGR of 4.97% during the forecast period. Remarkable growth of the market is driven by the rising integration of miniaturized molecular diagnostic platforms with smartphone-based interfaces, enabling portable, connected, and user-friendly testing for infectious diseases in decentralized and resource-limited environments.
Key Market Trends & Insights
- North America dominated the market with a revenue share of 53.47% in 2025.
- Asia Pacific is emerging as the fastest-growing region in the market, exhibiting a CAGR of 6.97% in 2025.
- By Product, the consumables segment dominated the market in 2025, accounting for 70.12% of the total share.
- By Technology, molecular diagnostics segment led the market with a revenue share of 46.78% in 2025.
- Based on Application, the respiratory infection testing products segment dominated the market with a 32.34% revenue share in 2025.
- Based on End Use, the hospital segment held the dominant share of 37.68% in 2025.
- The U.S. dominates the market, valued at USD 6.39 billion in 2024 and reaching USD 6.68 billion in 2025.
Table: U.S. Point of Care Infectious Disease Testing Market Share (USD Billion)
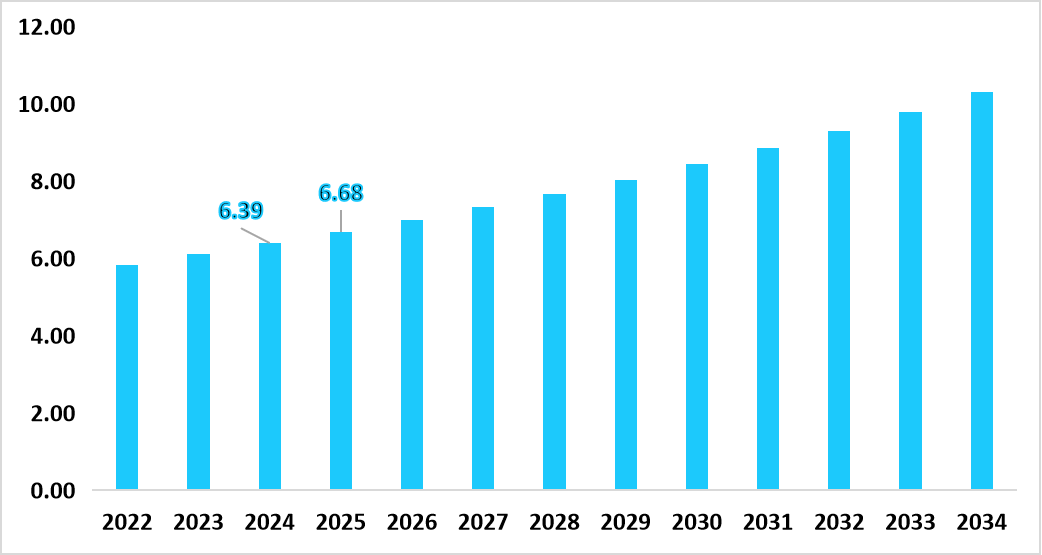
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 13.83 billion
- 2034 Projected Market Size: USD 21.32 billion
- CAGR (2026–2034): 4.97%
- Dominating region: North America
- Fastest-growing region: Asia Pacific
The point-of-care infectious disease testing market refers to a segment of the in-vitro diagnostics industry that focuses on providing rapid, accurate, and accessible detection of infectious pathogens directly at the site of patient care. These testing solutions minimize reliance on centralized laboratories by enabling timely diagnosis in hospitals, clinics, diagnostic laboratories, community health centers, and home-care settings. The market includes consumables such as reagents, assay strips, cartridges, and sample kits; instruments including portable analyzers and compact devices that automate detection processes; and software and services that integrate digital reporting, data analytics, and remote monitoring into clinical workflows. Based on technology, the market comprises lateral flow immunoassays, agglutination tests, flow-through or immunoconcentration assays, molecular diagnostic platforms, and other emerging biosensor-based systems that provide rapid and precise identification of infectious agents. In terms of application, POC testing is utilized for respiratory infections such as influenza and tuberculosis, healthcare-associated infections, tropical diseases including malaria and dengue, and sexually transmitted infections like HIV, hepatitis, syphilis, and human papillomavirus (HPV). The market serves diverse end users, including hospitals where testing is integrated into patient management systems, diagnostic laboratories that employ POC platforms for decentralized testing, home-care environments using self-test kits and connected devices, and other care settings such as urgent care and community health centers. Overall, the POC infectious disease testing market represents a critical advancement in modern diagnostics, combining molecular and immunoassay technologies with digital connectivity to improve disease surveillance, accelerate treatment decisions, and enhance global access to rapid diagnostic solutions.
Latest Market Trends
Shift from Laboratory-Based Testing to Decentralized and Home-Based Diagnostics
The global diagnostic ecosystem is experiencing a structural transformation as testing moves away from centralized laboratory facilities toward decentralized and patient-driven diagnostic models. This trend is fueled by the growing emphasis on rapid diagnosis, early intervention, and the demand for immediate clinical decision-making in community healthcare settings. Portable analyzers, disposable cartridges, and self-testing kits are now being deployed in clinics, pharmacies, and remote healthcare sites, enabling faster detection of infectious diseases such as influenza, HIV, and COVID-19. This transition has also been supported by the growing presence of retail diagnostic chains and pharmacy-led testing programs, which are extending diagnostic accessibility to underserved populations.
Shift from Manual Interpretation to Digitally Connected and AI-Enabled Platforms
The major transformation in the point of care infectious disease testing market is the shift from manually interpreted results toward digital, AI-enhanced diagnostic platforms. Traditional rapid tests often relied on visual colorimetric interpretation, which could vary based on operator experience or environmental factors. However, modern POC systems are increasingly incorporating AI algorithms, embedded sensors, and smartphone-compatible readers that automatically analyze test outcomes, reducing human error and improving diagnostic precision. Cloud connectivity is allowing results to be transmitted directly to healthcare databases or patient records, fostering seamless data exchange between clinicians, laboratories, and public health agencies. This trend is also driving large-scale disease surveillance initiatives, where aggregated diagnostic data can be used to track outbreak patterns and guide public health interventions in real time. The convergence of diagnostics, data analytics, and digital health is thus transforming POC testing from a standalone procedure into an intelligent, networked component of preventive and personalized healthcare systems.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 13.83 Billion |
| Estimated 2026 Value | USD 14.46 Billion |
| Projected 2034 Value | USD 21.32 Billion |
| CAGR (2026-2034) | 4.97% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Abbott, Hoffmann-La Roche Ltd , Thermo Fisher Scientific Inc., Siemens Healthineers AG, BD |
to learn more about this report Download Free Sample Report
Market Driver
Rising Global Burden of Infectious Diseases and Expansion of Preventive Screening Programs
The growing prevalence of infectious diseases remains one of the strongest forces driving demand for point-of-care diagnostics. Persistent outbreaks of respiratory infections, sexually transmitted diseases, and vector-borne illnesses have underscored the urgency of early and decentralized detection. Governments and health organizations worldwide are increasingly prioritizing preventive screening and outbreak preparedness, which has resulted in the large-scale adoption of portable testing solutions. National healthcare programs are integrating rapid diagnostic tools into surveillance frameworks to support immediate case identification and timely treatment initiation. For instance, the inclusion of rapid molecular and antigen-based POC tests in tuberculosis, malaria, and influenza control programs transformed the public health responsiveness, especially in low- and middle-income countries. This expanding focus on community-level screening and field-based diagnostics is propelling continuous market growth as healthcare systems seek to contain disease spread through faster, localized detection models.
Market Restraint
Variability in Test Accuracy Across Decentralized Settings
The point of care infectious disease testing market faces constraints related to test performance consistency. Variability in diagnostic accuracy across decentralized settings arises due to differences in operator proficiency, sample handling, and environmental factors such as temperature or humidity. These inconsistencies can lead to false positives or negatives, affecting clinical reliability and patient outcomes. Additionally, limited standardization across regulatory jurisdictions has resulted in discrepancies in product validation and quality assurance, particularly in emerging markets. The absence of uniform testing protocols and quality control mechanisms challenges global scalability, as healthcare providers must balance accessibility with diagnostic reliability. Addressing these limitations requires continuous product calibration, automated quality verification systems, and training initiatives for field healthcare personnel to maintain test precision in real-world applications.
Market Opportunity
Integration of POC Testing within Digital Health and Telemedicine Networks
The integration of point-of-care diagnostics with digital health and telemedicine ecosystems represents a major opportunity for market expansion over the next decade. The growing adoption of remote consultation models and digital patient management platforms is creating an interconnected diagnostic environment where test results can be shared instantly with healthcare professionals. This evolution enables continuous disease monitoring, timely therapeutic adjustments, and improved patient engagement, particularly in areas with limited physical healthcare access. Manufacturers are developing connected testing kits that synchronize with smartphone applications and electronic medical record (EMR) systems, allowing clinicians to interpret results remotely and initiate treatment protocols without delay. Furthermore, data collected from connected devices is enhancing epidemiological tracking, supporting policymakers and healthcare institutions in managing infectious disease trends. As healthcare delivery shifts toward virtual and hybrid care models, the fusion of POC testing with telehealth infrastructure is unlocking new pathways for real-time diagnostics, patient empowerment, and scalable disease management.
Regional Analysis
North America dominated the global market in 2025 with a revenue share of 53.47%, propelled by extensive diagnostic infrastructure, rapid adoption of molecular point-of-care platforms, and widespread screening initiatives for respiratory and sexually transmitted infections. Hospitals and clinics across the region are integrating decentralized testing models to reduce laboratory burden and improve patient turnaround time.
The U.S. market experienced steady growth driven by the adoption of multiplex molecular systems in primary care and emergency departments. Federal initiatives encouraging antimicrobial stewardship and early detection of community-acquired infections have intensified the use of rapid molecular assays. Continuous pipeline expansion from key players, coupled with FDA’s CLIA waiver approvals for multi-analyte assays, has positioned the U.S. as the core innovator in decentralized diagnostic adoption.
Asia Pacific Market Insights
The Asia Pacific region is projected to record the fastest growth of 6.97% through 2034, supported by evolving diagnostic landscapes, growing investment in portable testing platforms, and increased demand for decentralized healthcare delivery. Public–private collaborations are improving access to rapid diagnostics in community health centers and remote care environments. The market is also gaining traction due to local manufacturing initiatives that lower product costs and enhance regional supply resilience.
India’s market growth is driven by domestic manufacturing programs under initiatives promoting self-reliance in healthcare technology. Collaborations between national institutes and international diagnostics companies have expanded the availability of affordable molecular POC solutions for tuberculosis, dengue, and COVID-19 detection. Urban diagnostic chains are deploying cloud-linked rapid test kiosks for real-time disease surveillance, strengthening India’s role in advancing community-based infectious disease diagnostics.
Pie Chart: Regional Market Share, 2025
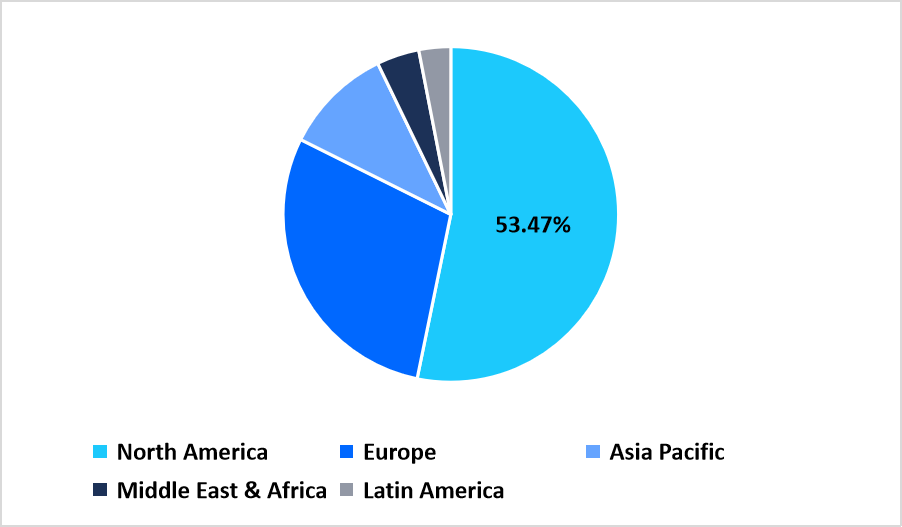
Source: Straits Research
Europe Market Insights
Europe maintains a strong presence in the POC infectious disease testing market due to its structured regulatory environment, clinical validation frameworks, and high testing volumes within national health systems. Emphasis on infection control, antibiotic resistance monitoring, and integration of POC data with electronic health records has created a mature ecosystem for rapid diagnostics. The region’s focus on sustainability and traceability in healthcare supply chains is further shaping procurement strategies for POC technologies.
The UK continues to expand its POC testing network through NHS-backed initiatives that enhance accessibility in primary care and pharmacy-led testing programs. Academic–industry partnerships are developing multiplex panels targeting respiratory and antimicrobial resistance markers. Adoption of connected diagnostic devices within integrated care systems is supporting early intervention models for infectious diseases and improving patient triage accuracy.
Middle East & Africa Market Insights
The Middle East & Africa region is observing gradual growth driven by government investments in laboratory decentralization and infectious disease preparedness programs. Strategic imports of portable analyzers and local assembly of rapid test kits are improving testing coverage across healthcare facilities. Partnerships with international diagnostics manufacturers are facilitating technology transfer and workforce training to address emerging infection surveillance challenges.
Saudi Arabia is advancing its POC testing capacity through integration of digital diagnostic platforms within national health transformation projects. Investments in locally adapted molecular and serological test production, combined with data-sharing collaborations with international health agencies, are improving public health responsiveness. The country’s commitment to healthcare digitization and disease outbreak readiness is strengthening its diagnostic infrastructure and regional influence.
Latin America Market Insights
Latin America’s market expansion is supported by improving access to affordable testing devices, increased awareness of early diagnosis, and regional collaborations for epidemic control. Public health agencies are promoting rapid diagnostic deployment in rural and peri-urban healthcare centers to manage infectious disease outbreaks efficiently. Local distributors are increasingly partnering with global players to scale availability and reduce dependency on imports.
Brazil’s diagnostic ecosystem is advancing through investments by public research institutions and private laboratories in near-patient molecular platforms. The adoption of POC assays for dengue, Zika, and respiratory infections is expanding in response to national health surveillance goals. Financial support from government-backed innovation funds is accelerating localization of diagnostic reagent production, establishing Brazil as a central hub for decentralized testing development in Latin America.
Product Insights
The consumables segment dominated the market in 2025, accounting for 70.12% of the total share. This dominance is attributed to the recurring demand for test strips, cartridges, and reagents used across diverse infectious disease assays. The growing adoption of multiplex kits and customized reagent formulations across decentralized healthcare facilities continues to sustain product replacement cycles and volume-driven revenue growth.
The software and services segment is projected to expand at the fastest CAGR of 5.76% during the forecast period. The surge in connected diagnostics, cloud-integrated data systems, and patient management software is transforming testing workflows by enabling real-time disease monitoring, analytics, and remote reporting. The integration of AI-based platforms for result interpretation is further driving digital transformation within point-of-care testing ecosystems.
Technology Insights
The molecular diagnostics segment led the market with a revenue share of 46.78% in 2025. The segment’s leadership stems from the extensive adoption of portable PCR and isothermal amplification systems capable of detecting multiple pathogens simultaneously. Continuous assay innovation for respiratory, sexually transmitted, and tropical infections has expanded clinical reach and enabled faster adoption across public health programs.
The lateral flow immunoassay segment is expected to grow at the fastest rate of 5.32% during the forecast period. Advancements in microfluidic strip design and visual readout accuracy are broadening applications in community screening and self-testing. Increasing product approvals for multiplex antigen and antibody assays are supporting transition toward low-cost, scalable diagnostic formats suited for large population testing.
Application Insights
The respiratory infection testing products segment dominated the market with a 32.34% revenue share in 2025. The widespread incidence of influenza, RSV, and emerging respiratory viruses has accelerated the deployment of rapid testing systems in outpatient and urgent care settings. Expansion of multiplex respiratory panels that identify multiple pathogens from a single sample has reinforced the clinical relevance of this segment.
The sexually transmitted disease (STD) testing products segment is anticipated to record the fastest growth rate of 5.24% during the forecast period. Innovations in molecular and immunoassay-based detection for HIV, hepatitis, and syphilis are expanding patient screening beyond laboratory settings. The increasing emphasis on early diagnosis, combined with decentralized testing models in reproductive and public health clinics, is propelling adoption across both developed and emerging economies.
End Use Insights
The hospital segment held the dominant share of 37.68% in 2025, driven by the integration of rapid molecular analyzers and connected diagnostic systems within clinical workflows. Large hospital networks are incorporating point-of-care devices for immediate pathogen detection, enabling faster clinical decision-making and optimized antibiotic stewardship. The presence of centralized procurement systems within hospitals enhances device standardization and long-term service contracts.
The home-care settings segment is projected to expand at the fastest CAGR of 5.89% through 2034. Rising consumer preference for self-testing kits, supported by smartphone-compatible diagnostic interfaces, is reshaping testing accessibility. The expansion of telehealth-based follow-up models and e-commerce distribution channels for POC kits is accelerating uptake in domestic healthcare environments.
Pie Chart: Segmentation by End Use in 2025 (%)
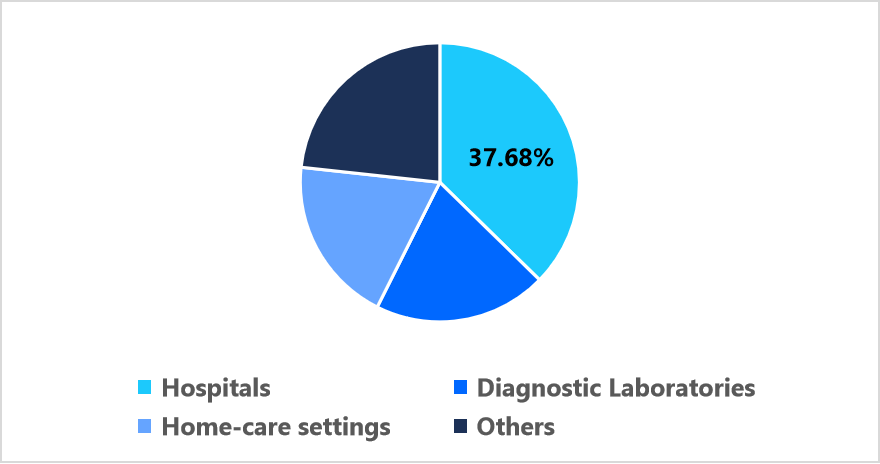
Source: Straits Research
Competitive Landscape
The global point-of-care (POC) infectious disease testing market is moderately fragmented owing to the presence of several multinational diagnostic companies and specialised solution providers offering a wide range of rapid testing platforms, molecular diagnostic analysers, and lateral flow assays.
Cepheid: An emerging market player
Cepheid established a strong leadership position in the POC infectious disease diagnostics space through its GeneXpert platform, which enabled rapid, PCR-based detection of pathogens such as SARS-CoV-2, tuberculosis, and HIV. The company continued to expand its multiplex respiratory and sexually transmitted infection (STI) test menus, integrating advanced cartridge technology that delivered lab-quality results within 30 minutes.
List of Key and Emerging Players in Point of Care Infectious Disease Testing Market
- Abbott
- Hoffmann-La Roche Ltd
- Thermo Fisher Scientific Inc.
- Siemens Healthineers AG
- BD
- Chembio Diagnostics, Inc.
- Trinity Biotech
- Cardinal Health
- Quest Diagnostics
- Bio-Rad Laboratories, Inc.
- BIOMÉRIEUX SA
- Trivitron Healthcare
- QuidelOrtho Corporation
- Cepheid
- Hologic, Inc
- OraSure Technologies Inc.
- EKF Diagnostics Holdings plc.
- Others
Strategic Initiatives
- October 2025: A research team led by Imperial College London developed a new low-cost, rapid diagnostic test called Dragonfly that detects asymptomatic cases of malaria from a simple finger-prick blood sample.
- July 2024: Hoffmann-La Roche Ltd completed the acquisition of LumiraDx’s point-of-care technology platform after receiving all required regulatory approvals. The platform included a multi-assay system capable of performing immunoassay, clinical chemistry, and coagulation tests. This acquisition further strengthened Roche’s leadership in the point-of-care infectious disease testing market, supporting its goal to expand decentralised diagnostic solutions and improve access to rapid and reliable testing across primary care and resource-limited settings.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 13.83 Billion |
| Market Size in 2026 | USD 14.46 Billion |
| Market Size in 2034 | USD 21.32 Billion |
| CAGR | 4.97% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Technology, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Point of Care Infectious Disease Testing Market Segments
By Product
- Consumables
- Instruments
- Software and Services
By Technology
- Lateral Flow Immunoassay
- Agglutination Test
- Flow-through test/ Immunoconcentration Assay
- Molecular Diagnostics
- Others
By Application
-
Respiratory Infection Testing Products
- Influenza testing products
- Tuberculosis
- Other respiratory infection testing products
- Healthcare-Associated Infection Testing Products
- Tropical Disease Testing Products
-
Sexually Transmitted Disease Testing Products
- HIV testing products
- Hepatitis testing products
- Syphilis testing products
- Human papillomavirus testing products
By End Use
- Hospital
- Diagnostic Laboratories
- Home-care settings
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































