Scleroderma Therapeutics Market Size, Share & Trends Analysis Report By Type (Localized Scleroderma, Systemic Sclerosis, Diffuse Sclerosis, Others), By Drug Class (Immunosuppressors, Phosphodiesterase 5 inhibitors – PHA, Endothelin Receptor Antagonists, Prostacyclin Analogs, Others), By Route of Administration (Parenteral, Oral, Topical), By End-User (Hospitals, Clinics, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Scleroderma Therapeutics Market Size
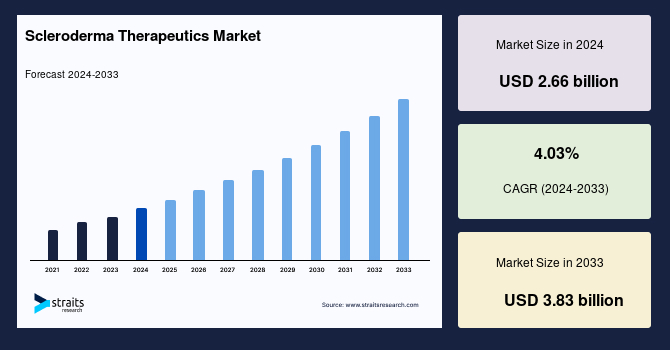
The global scleroderma therapeutics market size was valued at USD 2.66 billion in 2024 and is projected to grow from USD 2.79 billion in 2025 to reach USD 3.83 billion by 2033, exhibiting a CAGR of 4.03% during the forecast period (2025-2033).
Scleroderma therapeutics refer to medical treatments and therapies aimed at managing scleroderma, a chronic autoimmune disease that causes the skin and connective tissues to harden and tighten. The condition can affect internal organs as well, leading to complications in the heart, lungs, kidneys, and digestive system. Therapeutics for scleroderma include medications to control inflammation, suppress the immune system, and manage symptoms such as skin thickening, pain, and blood vessel problems.
The market is experiencing significant growth, driven by advancements in immunotherapies, anti-fibrotic treatments, and a deeper understanding of autoimmune diseases. As the global prevalence of scleroderma rises, particularly among women and adults, there is an increasing demand for effective treatments to manage complex symptoms such as fibrosis and lung involvement. The development of targeted therapies, including CAR T-cell therapies and biologics, is revolutionizing the management of systemic sclerosis and localized scleroderma.
- A notable example is the collaboration between the Scleroderma Research Foundation (SRF) and Sanofi, announced in April 2023, where Sanofi contributed its first experimental agent to CONQUEST, a clinical trial platform designed to expedite the development of promising treatments for scleroderma. This collaboration aims to identify which agents should advance from Phase 2b to Phase 3 trials, showcasing the critical role of partnerships in accelerating the search for effective therapies.
With growing awareness of scleroderma and its complications, coupled with expanding research and development in immunotherapy, the market is poised for continued growth. This expansion is further supported by regulatory approvals, government initiatives, and the increasing adoption of personalized treatment approaches, offering hope for improved patient outcomes.
Primary Key Market Trends
Shift toward Combination Therapies
The scleroderma therapeutics market is increasingly shifting toward combination therapies, recognizing that monotherapies often fall short in addressing the disease's complexity. By integrating treatments such as immunosuppressants, biologics, and antifibrotic agents, these approaches offer enhanced efficacy and help slow disease progression.
- For example, a study published in May 2024 in the National Library of Medicine highlighted the effectiveness of combining mycophenolate mofetil (MMF) and rituximab in treating progressive systemic sclerosis. The combination showed improved skin and lung function, with better event-free survival and safety compared to AHSCT over 24 months.
This shift toward combination therapies is driving research and expanding treatment options, ultimately improving outcomes for scleroderma patients.
Advancements in Diagnostic Techniques
Advances in diagnostic techniques are revolutionizing early disease detection, enabling more accurate and timely diagnoses. Innovations like nailfold capillaroscopy, high-resolution computed tomography (HRCT), and autoantibody profiling are improving diagnostic precision and facilitating better disease staging.
- For instance, in January 2024, a research team from the University of Alberta identified a new biological marker for scleroderma that allows for a simple blood test to predict disease severity. This breakthrough could dramatically improve early diagnosis and guide treatment strategies.
These advancements pave the way for personalized treatment approaches, ultimately enhancing patient outcomes and enabling earlier interventions in scleroderma management.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 2.66 Billion |
| Estimated 2025 Value | USD 2.79 Billion |
| Projected 2033 Value | USD 3.83 Billion |
| CAGR (2025-2033) | 4.03% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Hoffmann-La Roche AG, BLRBio, Corbus Pharmaceuticals Holdings, Inc., Calliditas Therapeutics AB., Celgene Corporation |
to learn more about this report Download Free Sample Report
Scleroderma Therapeutics Market Growth Factors
Rising Prevalence of Scleroderma
The increasing prevalence of scleroderma worldwide is a key driver of the scleroderma therapeutics market. A growing number of autoimmune disease cases, coupled with genetic and environmental risk factors, is leading to a higher incidence of scleroderma, fuelling the demand for effective treatments.
- For instance, in March 2023, an article published in the National Library of Medicine estimated that the global prevalence of systemic sclerosis (SSc) was 18.87 per 100,000 persons, affecting 1.47 million people. The study also found higher incidence and prevalence in females, adults, and high-income countries, highlighting the growing burden of the disease.
The rising prevalence is boosting the demand for innovative treatments, driving growth in the market.
Advancements in Immunotherapy and Anti-Fibrotic Drug Development
The development of immunotherapies and anti-fibrotic drugs is a significant driver in the market for scleroderma therapeutics. As scleroderma is characterized by immune system dysfunction and excessive fibrosis, targeted treatments aimed at modulating the immune response and preventing fibrosis are gaining traction.
- For instance, in February 2024, a study published in the National Library of Medicine explored the use of tocilizumab in systemic sclerosis-associated interstitial lung disease (SSc-ILD). The research found that tocilizumab significantly improved lung function and reduced disease progression, emphasizing the potential of targeted immunotherapies in managing complex scleroderma-related complications.
Such advancements in immunotherapy are driving growth in the market, improving patient outcomes and enhancing the potential for more effective treatments.
Market Restraining Factors
Limited Understanding of Disease Mechanism
A major challenge in the global scleroderma therapeutics market is the limited understanding of the disease's underlying mechanisms. Scleroderma, an autoimmune disorder, involves complex pathophysiology, and its exact causes remain poorly understood. This lack of clarity makes it difficult to identify effective biomarkers for diagnosis and targeted therapies for treatment. The absence of precise knowledge on how the disease progresses impedes the development of specific interventions that can effectively address its root causes, such as fibrosis and vascular damage. As a result, research and therapeutic innovation are slowed, creating barriers to finding optimal solutions for managing scleroderma.
Market Opportunity
Collaborative Research Initiatives
Collaborative research efforts between academic institutions, pharmaceutical companies, and healthcare providers offer a valuable opportunity to advance scleroderma research and treatment. By pooling resources, expertise, and data, these collaborations can accelerate the development of innovative therapies, improve disease understanding, and identify new biomarkers.
- For example, the October 2024 Genome Research in African American Scleroderma Patients (GRASP) study, a collaboration between Yale, Johns Hopkins University, and the National Human Genome Research Institute, aims to investigate genetic factors influencing scleroderma in African American patients. By collecting clinical data and blood samples, the study seeks to improve treatment strategies tailored to this population.
Collaborative efforts like these will significantly advance scleroderma research, facilitate the development of targeted therapies, and improve treatment outcomes for diverse patient groups.
Regional Insights
North America: Dominant Region with 39.8% Market Share
North America holds a dominant position in the global scleroderma therapeutics market, driven by a high rate of early diagnosis and increasing awareness of the disease. The region benefits from the presence of leading pharmaceutical companies, advanced research institutions, and a robust healthcare system. The widespread adoption of innovative therapies, including immunosuppressants and targeted biologics, is further accelerating market growth. Moreover, North America’s strong focus on clinical trials and cutting-edge treatment innovations solidifies its leadership, driving sustained demand for effective scleroderma therapies.
Asia Pacific: Fastest Growing Region with the Highest Market Cagr
Asia-Pacific is poised to register the fastest growth in the global scleroderma therapeutics market, with the highest projected CAGR. This growth is driven by increased awareness of autoimmune diseases, improved healthcare access, and rising demand for advanced treatment options in countries like Japan, China, and India. Government initiatives and expanding healthcare infrastructure are also contributing to the rapid adoption of novel therapies. As the region continues to invest in research and development, it is expected to play a pivotal role in shaping the future of scleroderma treatment, further fueling market expansion.
Countries Insights
- U.S.- The U.S. leads the scleroderma therapeutics market, benefiting from significant investments in research and development, along with strong regulatory support for clinical trials. In October 2023, Kyverna Therapeutics received FDA clearance for its third IND application for KYV-101, a CAR T-cell therapy targeting diffuse cutaneous systemic sclerosis. This approval paved the way for Phase 1/2 trials, underscoring the U.S.'s role in advancing innovative treatments for scleroderma.
- Germany- Germany is a key player in Europe’s scleroderma therapeutics market, driven by advanced treatment options and an increasing number of clinical trials. For example, Kyverna Therapeutics launched Phase 1/2 trials of KYV-101 in Germany in addition to the U.S., targeting diffuse cutaneous systemic sclerosis and lupus nephritis. Germany’s focus on clinical research and adoption of novel therapies enhances its position as a leader in scleroderma therapeutics in Europe.
- Canada- In Canada, the market growth is driven by rising investments in research and developments for scleroderma in the country. For instance, in 2022, BLR Bio received a USD 780,000 grant in collaboration with experts at the University of Saskatchewan, Canada, from the Canadian Institutes of Health Research to advance the study of BLR-200 in scleroderma. The ongoing research has shown promising in vivo results, highlighting the potential of BLR-200 as a therapeutic option for scleroderma.
- India– India’s scleroderma therapeutics market is experiencing rapid growth, driven by rising awareness of autoimmune diseases, expanding healthcare infrastructure, and increased access to advanced treatment options. Collaborations between local healthcare providers and international pharmaceutical companies are introducing innovative therapies, improving disease management, and enhancing patient outcomes. These factors are contributing to better diagnosis, improved treatments, and a growing focus on scleroderma care in India.
- Japan- Japan is experiencing significant growth in its market, driven by heightened awareness of autoimmune diseases and strong government support for healthcare innovation. The country’s robust research and development activities, particularly in immunotherapy and anti-fibrotic treatments, are introducing novel treatment options for scleroderma patients. Japan’s rapid adoption of new therapies and focus on clinical advancements contribute to its expanding role.
Segmentation Analysis
By Type
Systemic sclerosis is the largest segment in the global market, accounting for the highest revenue. This is due to its severe impact on multiple organ systems, including the lungs, heart, and kidneys, which often results in life-threatening complications. The complexity and multi-organ involvement increase the demand for specialized treatments, driving the market for effective therapies to manage these extensive challenges associated with systemic sclerosis.
By Drug Class
Immunosuppressors dominate the market for scleroderma therapeutics due to their ability to suppress the overactive immune system, thereby reducing inflammation and slowing disease progression. These drugs are essential in treating both localized and systemic sclerosis. By helping to control the immune response and prevent fibrosis, immunosuppressors are critical in managing scleroderma symptoms and minimizing complications, which makes them a cornerstone of treatment regimens for affected patients.
By Route of Administration
The oral route of administration holds the largest market share in the global market, primarily due to its ease of use and convenience for patients. Oral medications, such as immunosuppressors and endothelin receptor antagonists, are widely prescribed for managing systemic sclerosis and its complications. The non-invasive nature of oral treatments enhances patient adherence, offering a more accessible and patient-friendly approach to long-term disease management.
By End-User
Hospitals are the leading end-users in the global market, driving the highest market revenue. This is because hospitals are equipped with specialized medical teams and advanced diagnostic tools necessary for treating complex cases of systemic sclerosis, particularly those involving multi-organ complications. The infrastructure in hospitals supports the administration of complex therapies and provides comprehensive care for patients, making them the preferred healthcare setting for scleroderma treatment.
Company Market Share
Key players in the global scleroderma therapeutics industry are increasingly focusing on adopting a range of key business strategies to strengthen their position and drive growth. These strategies include strategic collaborations with academic institutions, healthcare providers, and other pharmaceutical companies to pool resources, knowledge, and expertise.
Amylyx Pharmaceuticals: An Emerging Player in the Global Scleroderma Therapeutics Market
AMYLYX Pharmaceuticals is dedicated to advancing therapies for rare diseases, with a particular focus on scleroderma. The company is committed to developing novel treatments that address the underlying mechanisms of fibrosis and inflammation, which are central to the progression of scleroderma. By targeting these key pathways, AMYLYX aims to offer potential breakthroughs that could significantly improve patient outcomes, especially for those experiencing severe complications related to the disease.
Recent developments by AMYLYX Pharmaceuticals:
- In January 2025, Amylyx Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) lifted the clinical hold on the Phase 1 trial of AMX0114, an investigational antisense oligonucleotide targeting calpain-2 for amyotrophic lateral sclerosis (ALS). This development marks a significant step forward, with Amylyx planning to open U.S. trial sites for screening, enrollment, and dosing.
List of Key and Emerging Players in Scleroderma Therapeutics Market
- Hoffmann-La Roche AG
- BLRBio
- Corbus Pharmaceuticals Holdings, Inc.
- Calliditas Therapeutics AB.
- Celgene Corporation
- Argentis Pharmaceuticals
- Kyverna Therapeutics
- Emerald Health Pharmaceutical
- Certa Therapeutics
- Bayer AG
- Sanofi
- Cytori Therapeutics Inc.
- Cabaletta Bio, Inc
- Prometic Life Sciences
- AMYLYX Pharmaceuticals

to learn more about this report Download Market Share
Recent Developments
- February 2025 – Certa Therapeutics announced that the U.S. Food and Drug Administration (FDA) granted Fast Track Designation for its investigational therapy FT011 for the treatment of systemic sclerosis (scleroderma). This follows the earlier granting of Orphan Drug Designation, highlighting the potential of FT011 in addressing unmet needs in the scleroderma therapeutics market and accelerating its development towards clinical use.
- March 2024 – Cabaletta Bio, Inc. announced that the U.S. FDA granted Orphan Drug Designation (ODD) to CABA-201, a fully human CD19-CAR T cell investigational therapy for the treatment of systemic sclerosis (SSc). This breakthrough is part of Cabaletta's RESET clinical trial program, which includes Phase 1/2 trials for autoimmune diseases driven by B cells, including the Phase 1/2 RESET-SSc trial, marking significant progress in developing targeted therapies for scleroderma.
Analyst Opinion
As per our analyst, the global scleroderma therapeutics market is on track for significant growth, spurred by breakthroughs in immunotherapies, anti-fibrotic drug development, and the increasing global prevalence of scleroderma. Precision medicine and targeted therapies, which focus on addressing the underlying mechanisms of scleroderma, are showing promising results in managing the complex and often debilitating complications of the disease.
The advent of novel treatment options, including CAR T-cell therapies and biologics, is driving further advancements in the market. Despite these advancements, the market faces key challenges, including the limited understanding of the disease’s exact mechanisms and the complexity of developing treatments that can effectively target multiple organ systems involved in systemic sclerosis.
Moreover, the high cost of treatment options and the variability in patient responses add additional hurdles in managing scleroderma. Nonetheless, collaborative research initiatives between pharmaceutical companies, academic institutions, and government entities are creating new opportunities to accelerate the development of effective therapies. The rise of emerging markets in Asia-Pacific and Europe presents additional growth opportunities.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 2.66 Billion |
| Market Size in 2025 | USD 2.79 Billion |
| Market Size in 2033 | USD 3.83 Billion |
| CAGR | 4.03% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Drug Class, By Route of Administration, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Scleroderma Therapeutics Market Segments
By Type
- Localized Scleroderma
- Systemic Sclerosis
- Diffuse Sclerosis
- Others
By Drug Class
- Immunosuppressors
- Phosphodiesterase 5 inhibitors – PHA
- Endothelin Receptor Antagonists
- Prostacyclin Analogs
- Others
By Route of Administration
- Parenteral
- Oral
- Topical
By End-User
- Hospitals
- Clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































