Syphilis Testing Market Size, Share & Trends Analysis Report By Type (Primary Syphilis, Secondary Syphilis), By Test Type (Non-treponemal tests (RPR, VDRL), Treponemal immunoassays (TPPA, TPHA), Enzyme immunoassays (ELISA) & chemiluminescent immunoassays (CLIA), Rapid diagnostic tests (lateral flow immunoassays), Molecular tests (PCR/NAAT), Multiplex STI panels, including syphilis targets), By Technology (Molecular Diagnostics, Immunoassay, Others), By Location of Testing (Laboratory Testing, Point of Care Testing) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Syphilis Testing Market Overview
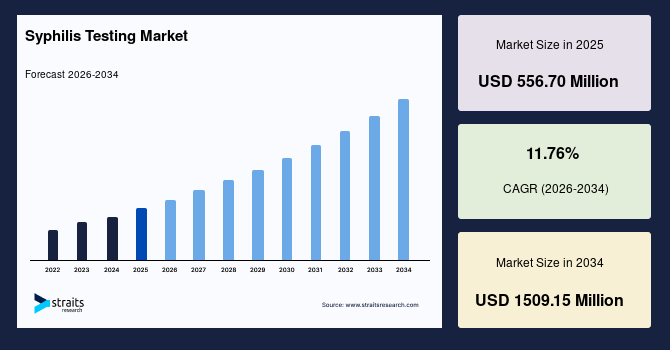
The global syphilis testing market size is estimated at USD 556.70 million in 2025 and is projected to reach USD 1509.15 million by 2034, growing at a CAGR of 11.76% during the forecast period. Remarkable growth of the market is propelled by the rising adoption of combined syphilis testing pathways that integrate rapid frontline screening with laboratory-based confirmation in a single diagnostic cycle.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 42.23% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.76%.
- Based on Type, the primary syphilis segment is anticipated to register the fastest CAGR of 12.12%.
- Based on Test Type, the Treponemal immunoassays (TPPA, TPHA) segment dominated the market growth with a revenue share of 35.63% in 2025.
- Based on Technology, the Immunoassay segment dominated the market growth in 2025 with a revenue share of 45.64%.
- Based on the Location of Testing, the point-of-care testing segment is anticipated to register the fastest CAGR of 12.67%.
- The U.S. dominates the global syphilis testing market, valued at USD 188.68 million in 2024 and reaching USD 21.0.06 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
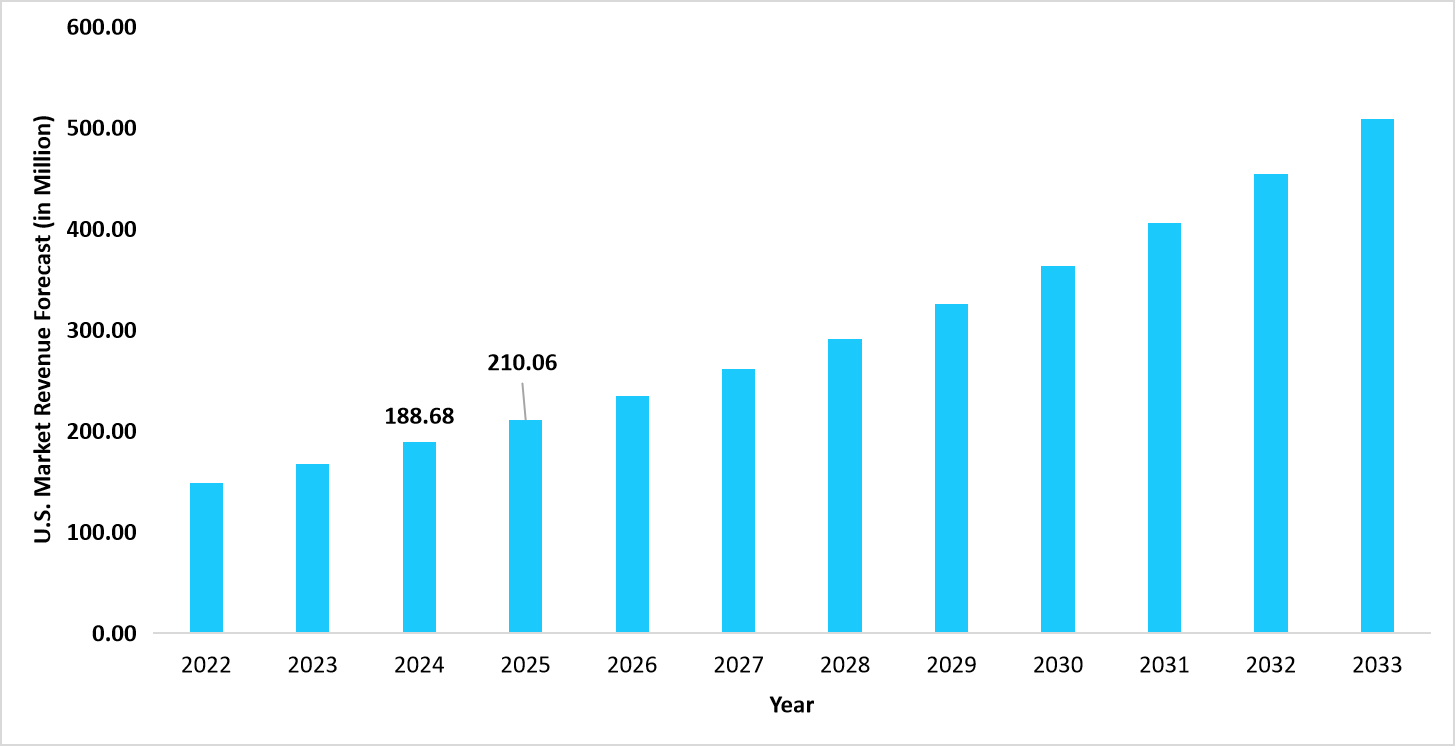
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 556.70 million
- 2034 Projected Market Size: USD 1509.15 million
- CAGR (2025 to 2034): 11.76%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The syphilis testing market comprises diagnostic products and services used to detect Treponema pallidum infection across various stages through laboratory-based evaluations and point-of-care screening methods. The market includes non-treponemal tests such as RPR and VDRL, treponemal immunoassays like TPPA and TPHA, enzyme immunoassays, chemiluminescent assays, rapid lateral flow formats, molecular tools such as PCR, and multiplex STI panels that incorporate syphilis targets. These tests are deployed across commercial laboratories, public health facilities, hospitals, and community-based clinics to support screening, diagnosis, monitoring, and management of syphilis in diverse population groups. Continuous attention toward early detection, rising adoption of structured screening pathways, and expansion of accessible testing sites drive ongoing development of diagnostic offerings within this market.
Latest Market Trends
Expansion of Dual Screening Models in Community Testing Programs
A rising trend in the syphilis testing market is the broader introduction of combined screening models where syphilis tests are paired with checks for other infections during single community visits. Clinics and outreach units are incorporating paired screening tables in local events, allowing individuals to complete multiple checks without additional appointments. This approach increases overall participation and encourages community groups to adopt structured testing routines. The trend gains traction as local health units explore ways to elevate turnout during routine screening days.
Adoption of Digital Triage Tools to Guide Test Selection
The major emerging trend is the rise of digital screening tools that support clinicians in selecting appropriate syphilis tests based on patient history, risk patterns, and symptom clusters. These platforms use structured questionnaires and clinical scoring prompts that guide medical teams toward the correct choice between rapid tests, treponemal assays, or laboratory-based evaluations. The growing use of such tools results in smoother decision pathways in clinics that manage large volumes of walk-in patients.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 556.70 Million |
| Estimated 2026 Value | USD 620.17 Million |
| Projected 2034 Value | USD 1509.15 Million |
| CAGR (2026-2034) | 11.76% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Hoffmann-La Roche Ltd, Abbott, BD, Siemens Healthineers AG, BIOMÉRIEUX |
to learn more about this report Download Free Sample Report
Market Driver
Rising Turnout in Community Screening Events Across Urban Centers
A key driver for the syphilis testing market is the expanding participation in community screening events operated by local health groups and clinics. Urban districts are hosting a growing number of open testing days where residents access syphilis checks without prior scheduling. This rising turnout widens the daily test volume for both rapid kits and laboratory panels, strengthening the use of structured screening campaigns as a core component of public health practice.
Market Restraint
Limited Uptake of Confirmatory Follow-Up Visits After Initial Testing
A primary restraint in the market is the low completion rate of confirmatory visits among individuals who receive preliminary test outcomes. A portion of patients do not return for secondary evaluation due to scheduling challenges, limited transportation, or poor awareness of follow-up steps. This lowers the total number of completed diagnostic pathways and reduces the overall clarity of case classification within health networks.
Market Opportunity
Growing Scope for Community-Based Syphilis Awareness Centers
A major opportunity is emerging through the expansion of community-based centers dedicated to year-round awareness and screening services. These centers can offer walk-in testing stations, informational sessions, and referral pathways for confirmatory examinations. As local groups invest in small-scale facilities within public areas, the presence of consistent testing access drives greater continuity in screening activities and expands the reach of syphilis detection services across diverse neighbourhoods.
Regional Analysis
North America maintained a leading position in the syphilis testing market with a share of 42.23% due to rising screening coverage across hospitals, community health centers, and outreach programs. The region recorded higher test volume as public health agencies expanded community-based screening drives, antenatal syphilis checks, and routine STI assessments. Growth was influenced by the broader deployment of point-of-care tests, the expansion of laboratory automation systems, and improved access to community clinics. Test suppliers continued to introduce assays that support rapid screening, confirmatory lab evaluation, and dual HIV syphilis formats, which encouraged wider adoption across public and private settings.
The U.S. market expanded as states scaled up syphilis testing through public health grants, mobile health units, and federally supported clinics. Demand increased as hospitals incorporated dual testing protocols for patients undergoing routine STI evaluation. Retail clinics and community centers made test access simpler, while electronic reporting systems supported stronger monitoring of regional test uptake. Commercial labs increased their syphilis assay offerings, creating higher throughput in urban and semi-urban regions.
Asia Pacific Market Insights
Asia Pacific recorded the fastest growth of 13.76% driven by rising awareness of congenital syphilis risks, enlargement of maternal care programs, and broader availability of screening tools in urban and semi-urban facilities. Public health authorities strengthened early pregnancy checks, resulting in higher use of both rapid tests and laboratory-based diagnostics. The expansion of online health platforms improved access to test information, encouraging higher participation in community screening efforts.
India’s market advanced as government hospitals added syphilis screening to antenatal visits and private diagnostic chains expanded STI panels. Urban clinics made rapid tests more accessible, while telehealth platforms increased awareness of early detection. Larger cities recorded rising participation in screening campaigns supported by non-profit groups and primary care networks.
Pie Chart: Regional Market Share, 2025
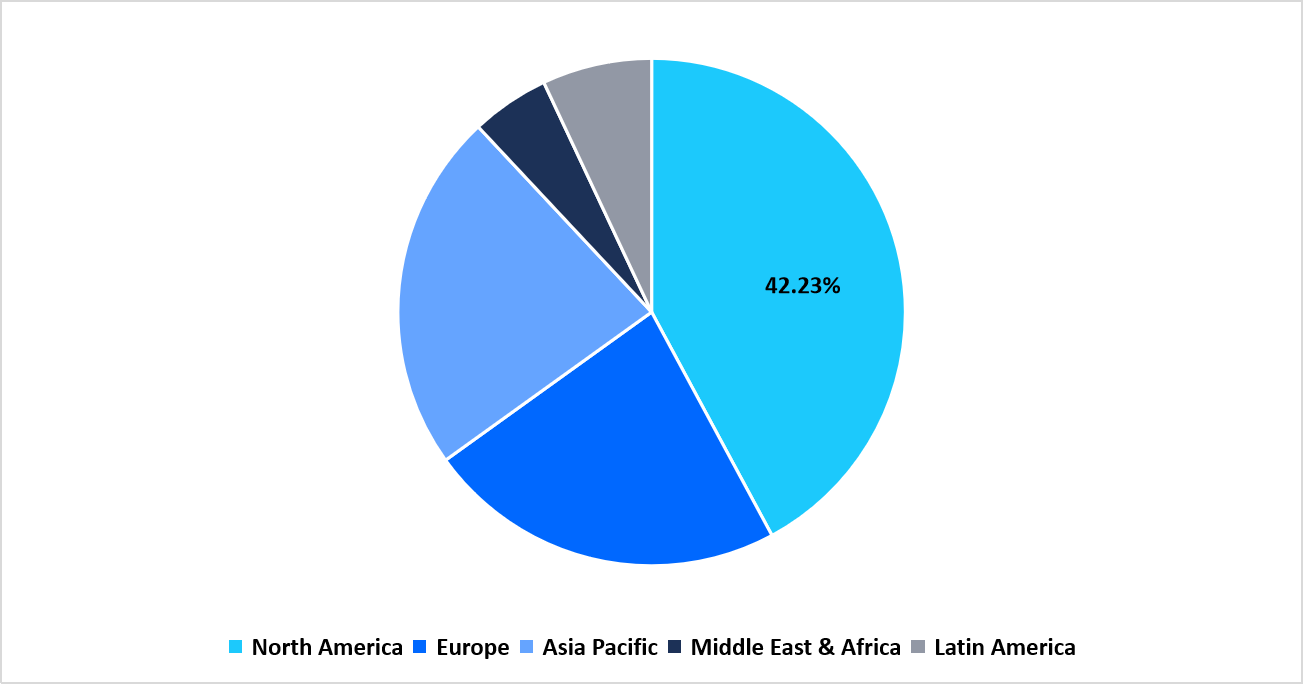
Source: Straits Research
Europe Market Insights
Europe moved ahead steadily as countries intensified STI surveillance activities and incorporated syphilis screening into routine diagnostic pathways. Hospitals adopted refined immunoassays for confirmatory evaluation, and primary care facilities increased basic screening checks. Growing interest in digital health reporting and structured public health initiatives contributed to consistent test adoption across multiple care settings.
Germany’s market gained traction as healthcare providers conducted systematic screening among individuals undergoing routine STI checks. Laboratory networks broadened treponemal and non-treponemal test offerings, increasing accessibility. Educational drives run by public health bodies contributed to higher turnout at clinics, driving consistent testing volume.
Middle East and Africa Market Insights
The Middle East and Africa region experienced rising uptake of syphilis testing as urban clinics strengthened STI diagnosis pathways and governments expanded maternal health screening. Imported rapid tests became more widely available through distributors, supporting greater usage in community health facilities. Public health workshops and awareness programs steadily increased recognition of early testing across several countries.
The United Arab Emirates market progressed as private hospitals added syphilis checks to routine sexual health assessments and travelers' screening programs. Diagnostic centers in Dubai and Abu Dhabi increased procurement of rapid tests and immunoassays, widening access. Wellness clinics and expatriate health providers contributed to a steady rise in testing adoption.
Latin America Market Insights
Latin America recorded ongoing progress supported by urban STI clinics, national antenatal care requirements, and government-backed surveillance projects. Community health units expanded access to rapid tests, while partnerships with international agencies improved the supply of diagnostic kits in several countries. Local distributors enhanced the availability of treponemal and non-treponemal assays across retail and clinic channels.
Brazil’s market grew as public hospitals strengthened maternal screening programs and community centers hosted regular walk-in testing days. Diagnostic laboratories adopted improved assay systems to manage rising test volumes. Urban areas recorded higher participation in voluntary testing events, reinforced by municipal outreach programs and medical universities promoting awareness of early detection.
Type Insights
Secondary syphilis dominated the market as diagnostic centers recorded higher detection rates among individuals presenting with extended symptoms during STI consultations. Clinics observed a steady inflow of patients requiring targeted evaluation for progressing infections, which supported a larger test count in this category. Consistent use of confirmatory examinations across hospitals maintained this segment in a leading position.
Primary syphilis is the fastest-growing segment, with 12.12% as early-stage detection increased across screening drives and routine checkups. Frontline facilities expanded the initial evaluation of high-exposure groups, resulting in more individuals being diagnosed during the first phase of infection. Wider participation in early testing programs supported the rapid advancement of this category.
Test Type Insights
Treponemal immunoassays dominated the segment with 35.63% due to broad use in confirmatory laboratory workflows where structured evaluation of suspected cases was required. Hospitals and private labs incorporated these assays into routine STI panels, antenatal testing pathways, and follow-up monitoring, ensuring higher volume across the year.
Rapid diagnostic tests is the fastest growing segment with 12.32% as outreach units, community clinics, and mobile programs expanded immediate screening across high traffic areas. Quick availability of test outcomes allowed on-site decision-making during community events and walk-in visits. Rising participation in local screening campaigns strengthened growth in this category.
Technology Insights
Immunoassay dominated the market with 45.64% as laboratories used these systems for large-scale screening and confirmatory evaluation. Diagnostic networks relied on established platforms to process daily sample loads from clinics and public health centers. Strong presence across regional hospitals kept this category ahead of others.
Molecular diagnostics is the fastest-growing segment, with 12.45% driven by increasing use in the detailed evaluation of complex cases and high-risk patients. Facilities conducting expanded STI panels incorporated molecular tools for precise detection of early-stage infections. Greater placement of these systems in select urban labs supported upward growth.
Segmentation by Technology in 2025 (%)
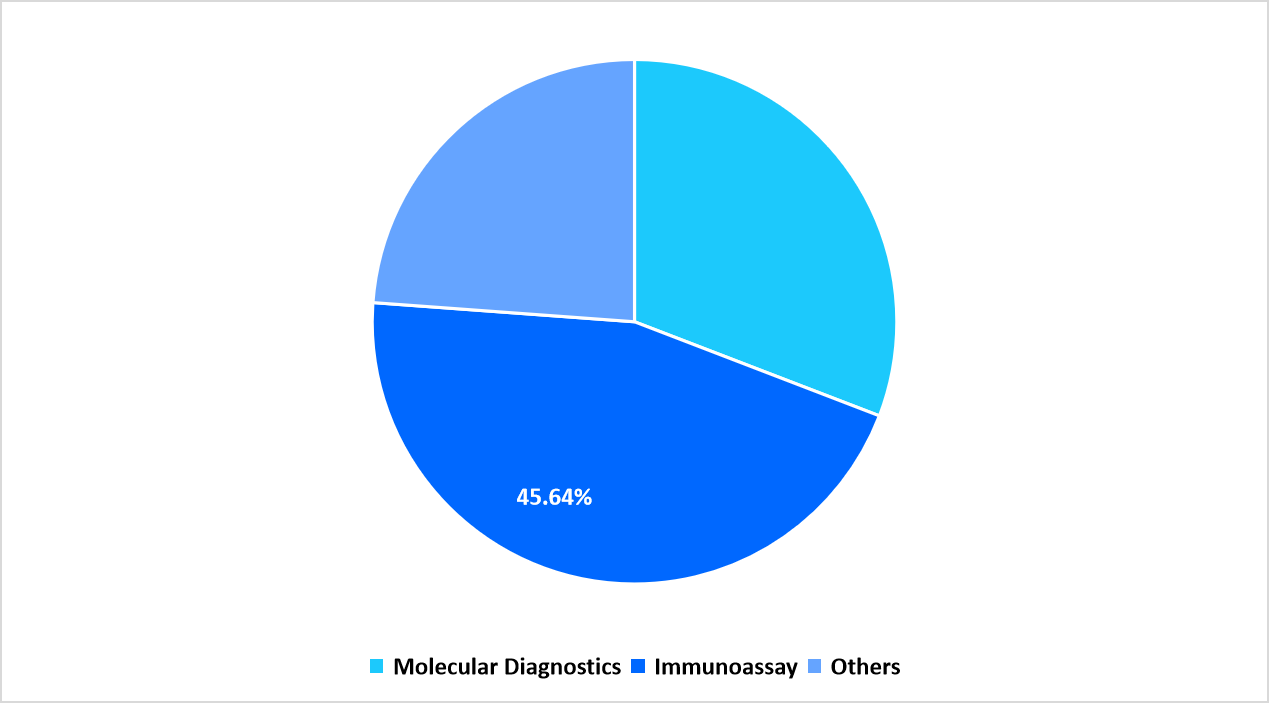
Source: Straits Research
Location of Testing Insights
Laboratory testing dominated the market as hospitals, reference labs, and public facilities conducted structured evaluations for both treponemal and non-treponemal markers. These centers managed steady volumes from outpatient units, maternity clinics, and community referral programs, keeping lab-based testing at the forefront.
Point of care testing is the fastest growing segment, with 12.67% as walk-in clinics, outreach groups, and field units expanded instant screening availability. Broader distribution of rapid kits enabled easier access for individuals seeking immediate testing. Participation in community-level drives pushed this category upward at a strong pace.
Competitive Landscape
The syphilis testing market is moderately fragmented with a combination of established global diagnostics manufacturers and a rising group of agile innovators specializing in rapid point-of-care screening, multiplex infectious disease testing, and community-level diagnostic access.
BioLytical Laboratories Inc.: An Emerging Market Player
- BioLytical Laboratories Inc. gained recognition as an innovative entrant in the syphilis testing space through its ultra-rapid point-of-care diagnostic technologies. The company is known for developing rapid tests designed to deliver results within minutes, improving the speed and reach of community-based screening programmes. bioLytical focused on expanding its INSTI platform to include syphilis and combined HIV syphilis formats, enabling integrated testing during outreach drives, antenatal care screening and high-volume public health campaigns. Its emphasis on fast turnaround time, ease of use, and deployment in low-resource settings positioned the company as a fast-growing competitor in the global syphilis testing landscape.
List of Key and Emerging Players in Syphilis Testing Market
- Hoffmann-La Roche Ltd
- Abbott
- BD
- Siemens Healthineers AG
- BIOMÉRIEUX
- Hologic, Inc.
- Cepheid
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific Inc
- QuidelOrtho Corporation
- Trinity Biotech
- SD Biosensor, INC
- ARLINGTON SCIENTIFIC
- DiaSorin S.p.A.
- Beckman Coulter, Inc.
- bioLytical Laboratories Inc.
- Others
Strategic Initiatives
- November 2025: The Delaware County Public Health Department in Iowa partnered with UnityPoint Health VNA to host free walk-in public clinics that provided syphilis testing. The initiative offered confidential and easily accessible syphilis screening in a community setting. This development expanded the range of testing venues, including community clinics and outreach programs, which increased testing volume and supported broader adoption of point-of-care syphilis tests.
- July 2025: The World Health Organization announced the rollout of an integrated testing model in the Democratic Republic of the Congo that combined syphilis and HIV rapid testing with mpox diagnostic services. Between April and early June 2025, 697 individuals suspected of mpox were tested for HIV and syphilis at pilot sites, and six of them were found to have syphilis and were treated at the site. The initiative was planned for a national scale-up. This development demonstrated how integrated testing models expanded syphilis testing access through shared platforms and service channels, which created new opportunities for multiplex and co-packaged diagnostic solutions.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 556.70 Million |
| Market Size in 2026 | USD 620.17 Million |
| Market Size in 2034 | USD 1509.15 Million |
| CAGR | 11.76% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Test Type, By Technology, By Location of Testing |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Syphilis Testing Market Segments
By Type
- Primary Syphilis
- Secondary Syphilis
By Test Type
- Non-treponemal tests (RPR, VDRL)
- Treponemal immunoassays (TPPA, TPHA)
- Enzyme immunoassays (ELISA) & chemiluminescent immunoassays (CLIA)
- Rapid diagnostic tests (lateral flow immunoassays)
- Molecular tests (PCR/NAAT)
- Multiplex STI panels, including syphilis targets
By Technology
- Molecular Diagnostics
- Immunoassay
- Others
By Location of Testing
-
Laboratory Testing
- Commercial/Private Labs
- Public Health Labs
- Point of Care Testing
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































