T-Cell Acute Lymphoblastic Leukemia Treatment Market Size, Share & Trends Analysis Report By Treatment Type (Chemotherapy, Targeted therapy, Radiation Therapy, Bone Marrow Transplant), By End User (Hospitals & Clinics, Cancer Care Centers, Research and Academic Institutes) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
T-Cell Acute Lymphoblastic Leukemia Treatment Market Overview
The global T-cell acute lymphoblastic leukemia market size is valued at USD 2.10 billion in 2025 and is estimated to reach USD 3.71 billion by 2034, growing at a CAGR of 6.57% during the forecast period. The growth of the T-cell acute lymphoblastic leukemia market is accelerated by advanced models that replicate a patient’s real tumor environment, allowing researchers to assess drug responses more precisely, tailor personalized treatment strategies, and expedite the development of targeted therapies.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 39.76% share in 2025.
- The Asia Pacific region is forecasted to grow at the fastest pace, with a CAGR of 62%.
- Based on treatment type, the chemotherapy segment dominated the market, accounting for a revenue share of 34.52% in 2025.
- Based on end user, the cancer care centers segment is expected to register the fastest CAGR of 7.03% during the forecast period.
- The U.S. dominates the market, valued at USD 737.34 million in 2024 and reaching USD 782.61 million in 2025.
Table: U.S. T-cell Acute Lymphoblastic Leukemia Market Size
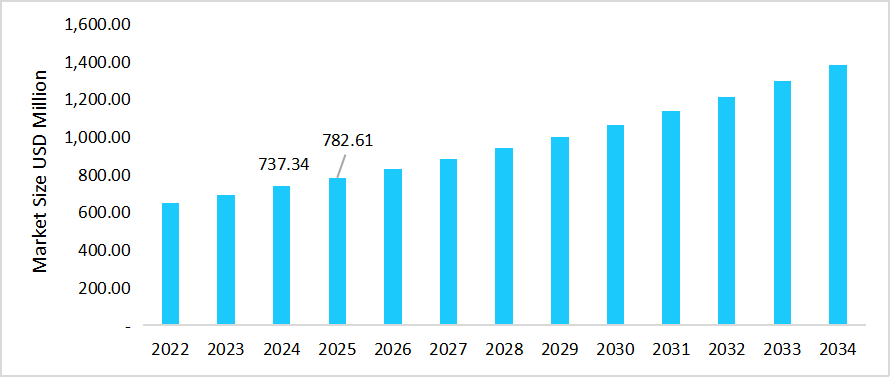
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.10 billion
- 2034 Projected Market Size: USD 3.71 billion
- CAGR (2026-2034): 6.57%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The global T-cell acute lymphoblastic leukemia market includes a range of therapeutic approaches, such as chemotherapy, targeted therapy, radiation therapy, and bone marrow transplantation, each tailored to manage different stages and complexities of the disease. These treatments are delivered through various healthcare settings, such as hospitals and clinics, cancer care centers, and research and academic institutes, providing comprehensive patient care.
Market Trends
Expansion of CAR T-cell and Targeted Therapy Pipelines
According to Straits Research, a growing emphasis on CAR T-cell therapies and targeted treatments is a key trend in the T-cell acute lymphoblastic leukemia treatment market. Traditionally, chemotherapy dominated T-ALL treatment; however, the focus is gradually shifting toward precision oncology and patient-specific therapies. Leading pharmaceutical companies, including Novartis and Pfizer, have advanced multiple CAR T-cell therapy candidates for T-ALL into phase 3 clinical trials, reflecting remarkable investment in novel treatment options.
This underscores the emergence of immunotherapies as a major trend in the market.
Rising Collaborations between Biotech Firms and Academic Institutions
A key trend in the T-cell acute lymphoblastic leukemia treatment market is the growing collaboration between pharmaceutical companies, biotech firms, and academic institutions to accelerate the discovery and development of innovative therapies. These partnerships combine industry resources with academic research expertise, speeding up innovation and clinical translation.
- For example, in November 2023, Arcellx and Kite Pharma entered a global strategic collaboration to co-develop and commercialize CART-ddBCMA for lymphoma treatment.
Such collaborations are streamlining drug development, strengthening pipelines, and fueling overall market growth in T-ALL therapies.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.10 Billion |
| Estimated 2026 Value | USD 2.23 Billion |
| Projected 2034 Value | USD 3.71 Billion |
| CAGR (2026-2034) | 6.57% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Bristol Myers Squibb, Gilead Sciences, Novartis AG, Pfizer Inc., Hoffmann-La Roche Ltd. |
Market Drivers
Increasing Prevalence of T-cell Acute Lymphoblastic Leukemia
The rising incidence of T-cell acute lymphoblastic leukemia is emerging as a major growth driver for the T-ALL treatment market. According to the American Cancer Society, approximately 6,000 new cases of acute lymphoblastic leukemia are diagnosed annually in the U.S., with T-ALL representing a substantial proportion, while the global incidence continues to gradually increase. This increased prevalence fueled the demand for advanced therapies, including targeted treatments and immunotherapies, thereby supporting market expansion and driving the adoption of novel treatment options worldwide.
Market Restraint
High Relapse Rates in T-cell Acute Lymphoblastic Leukemia Treatment
A major restraint in the T-cell acute lymphoblastic leukemia treatment market is the high relapse rate, which continues to limit long term patient outcomes despite advances in therapies. Clinical studies published in Blood report stated that relapse occurred in nearly 40–50% of adult T-ALL patients, with poor prognosis.
This recurring challenge reduced the overall potency of both conventional and novel therapies, hindering adoption and slowing market growth as companies struggle to address the persistent risk of relapse.
Market Opportunity
Development of Novel Bispecific Antibodies for T-ALL
The advancement of bispecific antibody therapies designed to improve treatment efficacy and address resistance challenges is a key opportunity for market growth. For example, in 2024, new clinical trials launched to evaluate CD3 and CD7-targeted bispecific antibodies, which demonstrated promising anti-leukemic activity in refractory T-ALL patients.
Such inventions created a new scope for pharmaceutical companies to expand their pipelines, accelerate commercialization, and deliver innovative therapies, thereby improving patient outcomes.
Regional Analysis
North America dominated the market in 2025, accounting for 39.76% market share. This growth is supported by the availability of accelerated regulatory pathways such as the FDA’s breakthrough therapy and regenerative medicine designations, which shortened approval timelines for novel T-ALL therapies and encouraged faster commercialization. In addition, the region’s dense network of academic medical centers actively conducts CAR-T and immunotherapy clinical trials. These combined advantages drive innovation, adoption, and sustained leadership of North America in the T-ALL treatment market.
In Canada, the launch of the country’s first fully “made-in-Canada” CAR T therapy reduced the reliance on international supply chains, cutting down manufacturing delays and improving patient access to advanced treatments. In addition, Health Canada introduced supportive guidelines tailored for cell and gene therapies, enabling faster product approvals and commercialization. These combined efforts are driving market growth in Canada.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 8.62% from 2026-2034, owing to the establishment of advanced cell and gene therapy manufacturing hubs, such as Singapore’s ACTRIS facility, which provides Good Manufacturing Practices-compliant labs and clean rooms to accelerate production of innovative therapies. In addition, China’s NMPA approvals for domestic CAR-T therapies and South Korea’s Advanced Regenerative-Bio Act are streamlining commercialization and strengthening the regional T-ALL treatment landscape.
India's T-cell acute lymphoblastic leukemia treatment market is gaining momentum with unique domestic advancements. The launch of NexCAR19, the country’s first homegrown CAR-T therapy, delivered high response rates while being priced at USD 33,813-35,000, notably cheaper than international alternatives, improving affordability for patients. In addition, Bharat Biotech’s $75 million investment in a new cell and gene therapy facility in Genome Valley is boosting local manufacturing capacity and reducing import reliance, thereby supporting market growth.
Regional Market share (%) in 2025

Source: Straits Research
Europe Market Insights
Europe's T-cell acute lymphoblastic leukemia treatment market growth is supported by the adoption of centralized precision oncology centers, which consolidate genomic testing and advanced therapy administration under one roof, improving treatment efficiency. Additionally, Europe’s robust health technology assessment (HTA) frameworks encourage reimbursement of innovative therapies, accelerating patient access to T-ALL treatments and boosting overall market adoption.
Germany's T-cell acute lymphoblastic leukemia treatment market growth is driven by strong public healthcare coverage under the statutory health insurance (SHI) system, which ensures patient access to costly oncology treatments. Additionally, the German Society of Hematology and Oncology (DGHO) updated its leukemia treatment guidelines to include novel targeted and immunotherapy options such as CAR T-cell therapy and bispecific antibodies for relapsed or refractory T-ALL patients. These factors collectively boost the market growth in Germany.
Latin America Market Insights
The growth of the T-cell acute lymphoblastic leukemia treatment market in Latin America is supported by growing participation in international compassionate use and early access programs. This approach not only improves patient survival outcomes but also strengthens regional collaboration with global biopharma companies, fostering early technology transfer and accelerating therapeutic innovation in the region.
Brazil's T-ALL treatment market growth is stimulated by the government’s investment in decentralized cell therapy production units within public hospitals. This initiative reduces logistical delays and treatment costs associated with centralized manufacturing, which leads to faster access to CAR-T and gene therapies for patients across diverse regions, thereby strengthening Brazil’s position in advanced oncology care.
Middle East and Africa Market Insights
The key factor driving market growth in the Middle East and Africa region is the increasing awareness of hematologic cancers through national cancer education programs and public health campaigns. These initiatives are improving early diagnosis rates, encouraging timely treatment initiation, and fostering acceptance of advanced therapies, thereby accelerating the overall adoption of T-ALL treatments across the region.
South Africa’s T-cell acute lymphoblastic leukemia treatment market growth is driven by the expansion of public private partnerships focused on developing regional oncology training hubs. These programs enhance the skills of local oncologists and laboratory specialists in advanced cell and gene therapies, improving treatment delivery standards and strengthening the country’s capacity for complex leukemia care.
Treatment Type Insights
The chemotherapy segment dominated the market, accounting for a revenue share of 34.52% in 2025. This growth is driven by its role as a bridge therapy for patients awaiting advanced treatments like CAR-T. Since cell therapies require time for manufacturing, chemotherapy remains the immediate option to control disease progression, ensuring patient stability until novel therapies become available.
The targeted therapy segment is projected to grow at the fastest CAGR of 7.72% during the forecast period, due to the development of precision biomarkers that enable personalized treatment regimens and minimize off-target effects. Moreover, improved drug delivery technologies, such as nanoparticle-based carriers, enhance therapeutic efficacy and reduce systemic toxicity, making targeted therapies a preferred choice for T-ALL management.
Treatment Type Market Share (%), 2025
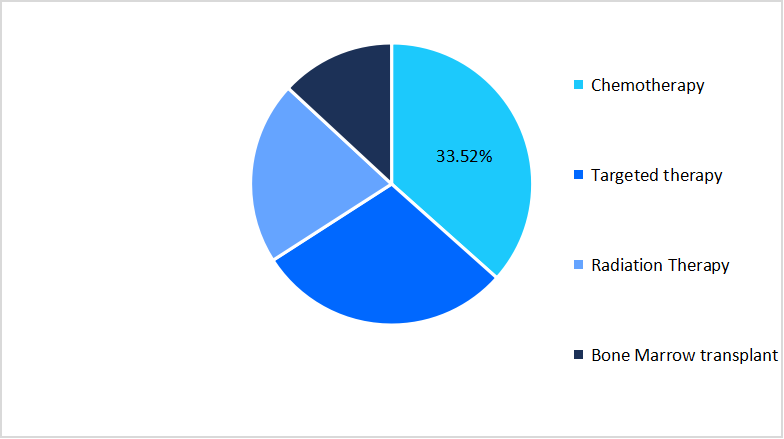
Source: Straits Research
End User Insights
The hospitals and clinics segment dominated the market, accounting for 48.37% in 2025. This dominance is augmented by the adoption of multidisciplinary tumor boards. By bringing together oncologists, hematologists, radiologists, and genetic counselors, these boards facilitate collaborative treatment planning, optimize therapy selection for T-ALL patients, and improve overall care quality, thereby boosting the segment’s market prominence.
The cancer care centers segment is estimated to register the fastest CAGR of 7.03% during the forecast period, owing to specialized infusion facilities within these centers that enable safe administration of complex therapies such as bispecific antibodies, which community clinics often cannot provide. Further, the integration of genomic profiling labs directly within cancer centers accelerates personalized treatment planning, reducing delays between diagnosis and therapy initiation, thereby strengthening patient preference and market growth.
Competitive Landscape
The global T-cell acute lymphoblastic leukemia treatment market is highly consolidated, with a few leading companies holding dominant positions. Key players include Novartis AG, Pfizer Inc., F. Hoffmann-La Roche Ltd., Bristol-Myers Squibb, Amgen Inc., Gilead Sciences (Kite Pharma), and others.
These companies are actively focusing on the clinical development of bispecific antibodies, while also pursuing strategic collaborations, acquisitions, and R&D investments to strengthen their pipelines, expand geographic reach, and maintain a competitive edge in this specialized oncology segment.
Autolus Therapeutics: An emerging market player
Autolus Therapeutics is an emerging clinical-stage biopharmaceutical company specializing in the development of advanced CAR T-cell therapies for hematological cancers, including T-cell acute lymphoblastic leukemia.
- In 2024, the company progressed its lead candidate, obe-cel (AUTO1), a CD19-targeted CAR T-cell therapy, into late stage clinical trials, demonstrating promising efficacy in patients with relapsed or refractory acute lymphoblastic leukemia and highlighting its potential to address high unmet medical needs in hematologic malignancies.
List of Key and Emerging Players in T-Cell Acute Lymphoblastic Leukemia Treatment Market
- Bristol Myers Squibb
- Gilead Sciences
- Novartis AG
- Pfizer Inc.
- Hoffmann-La Roche Ltd.
- Kyowa Kirin Co., Ltd.
- Genmab A/S
- Amgen Inc.
- Merck & Co., Inc.
- Spectrum Pharmaceuticals
- Sanofi S.A.
- Johnson & Johnson Services Inc.
- Others
Strategic Initiatives
- July 2025: Autolus Therapeutics plc, announces today that the European Commission (EC) has granted marketing authorization for AUCATZYL (obecabtagene autoleucel ) for the treatment of adult patients with acute lymphoblastic leukemia.
- November 2024: The Food and Drug Administration approved Autolus’s obecabtagene autoleucel, a CD19-directed genetically modified autologous T cell immunotherapy, for adult patients with acute lymphoblastic leukemia.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.10 Billion |
| Market Size in 2026 | USD 2.23 Billion |
| Market Size in 2034 | USD 3.71 Billion |
| CAGR | 6.57% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Treatment Type, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
T-Cell Acute Lymphoblastic Leukemia Treatment Market Segments
By Treatment Type
- Chemotherapy
- Targeted therapy
- Radiation Therapy
- Bone Marrow Transplant
By End User
- Hospitals & Clinics
- Cancer Care Centers
- Research and Academic Institutes
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































