Antibody Drug Conjugates Market Size, Share & Trends Analysis Report By Product Type (Adcetris, Kadcyla, Enhertu, Padcev, Trodelvy, Others), By Application (Blood Cancer, Breast Cancer, Urothelial Cancer, Lung Cancer, Others), By Target (CD33, HER2, CD30, TROP2, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Antibody Drug Conjugates Market Overview
The global antibody drug conjugates market size is valued at USD 13.63 billion in 2025 and is anticipated to grow from USD 15.41 billion in 2026 to USD 34.87 billion by 2034, growing at a CAGR of 11.04% from 2026 to 2034. Increasing approvals of HER2-targeted therapies, strategic ADC development partnerships across Asia Pacific, and rising adoption of antibody drug conjugates for cancer treatment are driving robust growth in the market.
Key Market Trends & Insights
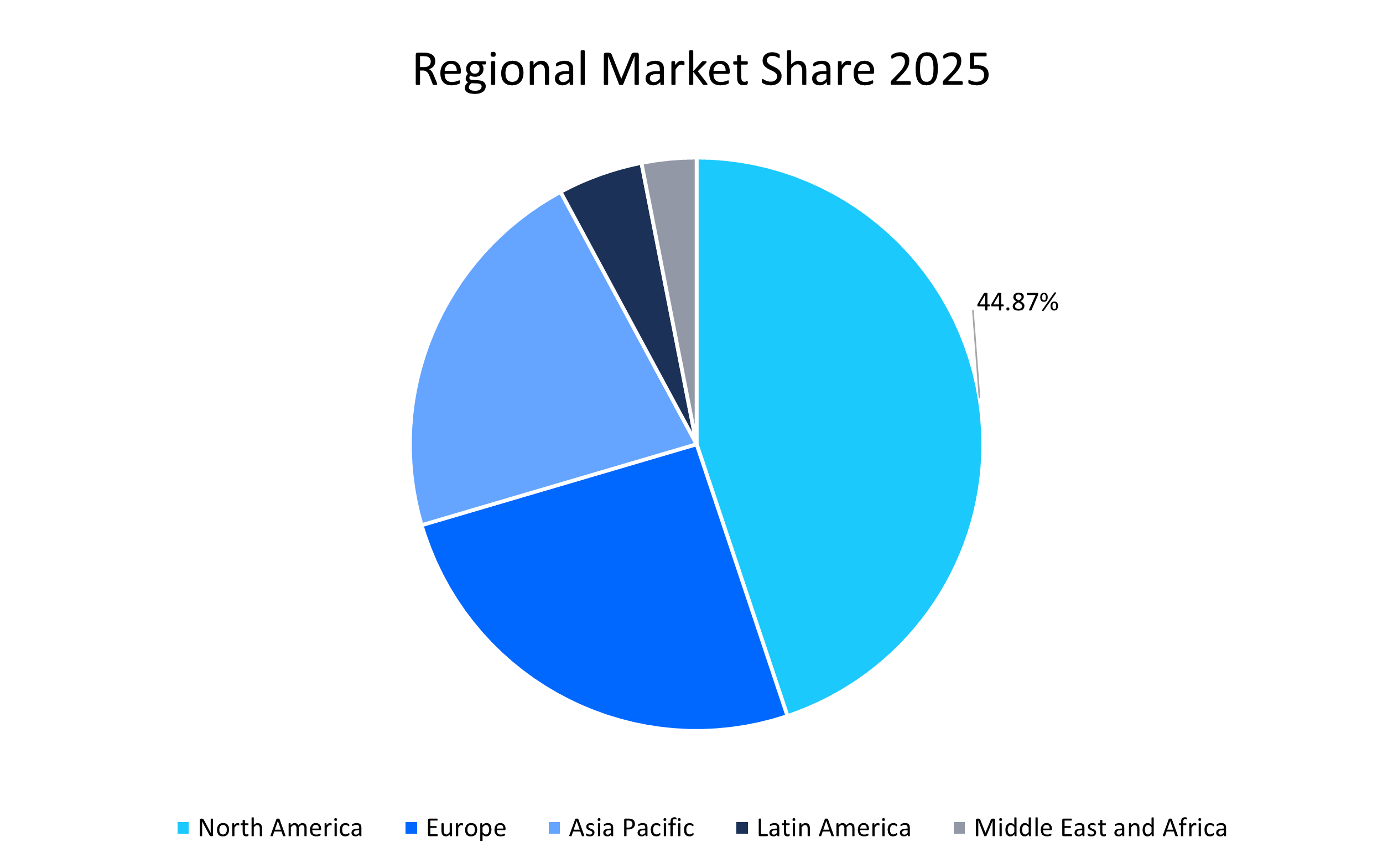
- North America held a dominant share of the global market, accounting for 44.87% share in 2025, due to its advanced R&D capabilities and increased consumption of ADCs.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 12.7%, due to rising cancer prevalence, healthcare investments, and growing merger and acquisition activities.
- Based on product type, the Kadcyla segment held the highest market share of 28.86% in 2025. This is attributed to the highest number of product approvals of Kadcyla.
- On the basis of application, breast cancer is expected to register the fastest CAGR growth of 12.4%, owing to rising approvals of targeting lung cancer and a surge in prevalence of lung cancer across the globe.
- The U.S. dominates the antibody drug conjugates market, valued at USD 5.24 billion in 2024 and reaching USD 5.80 billion in 2025.
Market Size and Forecast
- 2025 Market Size: USD 13.63 billion
- 2034 Projected Market Size: USD 34.87 billion
- CAGR (2026-2034): 11.04%
- Dominating Region: North America
- Fastest-Growing Region: Asia-Pacific
Antibody drug conjugates are becoming highly valuable in the market, with currently 15 approved ADCs targeting 16 different indications for hematological and solid tumor malignancies as of June 2025. Pfizer Inc., AstraZeneca, Gilead Sciences, F. Hoffmann-La Roche Ltd., along with other pharmaceutical companies, are significantly investing in ADCs. Thus, market consolidation is a major driver boosting the revenue of global manufacturers.
Table: Top 5 Antibody Drug Conjugate Manufacturers
|
Manufacture |
ADC |
Revenue 2024 (USD Million) |
|
F. Hoffmann La-Roche Ltd. |
Kadcyla |
2,270.9 |
|
Polivy |
1,274.1 |
|
|
Pfizer Inc. |
Adcetris |
1,089.0 |
|
Padcev |
1,588.0 |
|
|
AstraZeneca |
Enhertu |
1,982.0 |
|
Gilead Sciences |
Trodelvy |
1,315.0 |
|
AbbVie Inc. |
Elahere |
479.0 |
Source: Company Annual Reports & Straits Analysis

Source: Straits Research Analysis
Market Trends
Frontline Use of Enhertu in the U.s.
The use of Enhertu through advancing payloads is a key trend in the U.S. market. For example, Enhertu with pertuzumab has delivered a 40.7-month progression-free survival compared to 26.9 months for standard chemotherapy. This showcases ADCs as a potential first line treatment of care in HER2+ metastatic breast cancer across North America.
This fuels the demand for Enhertu in North America, which, in turn, contributes to the market growth.
Key Players' Consolidation Strategies
Global manufacturers are taking the ADC market towards consolidation by acquiring smaller companies to advance their clinical cancer pipelines. This highlights the importance of ADC innovation in the U.S.
- For example, in 2024, AbbVie acquired ImmunoGen to add ELAHERE to its cancer product portfolio. This acquisition proved highly beneficial for AbbVie as it generated USD 479 million in 2024, making AbbVie one of the key players in the antibody drug conjugates market.
Elahere 2024 Journey
|
Date |
Elahere Development |
|
November 2024 |
CE grant marketing authorization to treat adult patients with FRα-positive, serious epithelial ovarian and fallopian tube cancer. |
|
June 2024 |
Positive phase 2 results from PICCOLO trial, where Elahere was evaluated as monotherapy in patients with platinum-sensitive ovarian cancer. |
|
March 2024 |
U.S. FDA approval for the indication of epithelial ovarian and fallopian tube cancer |
Source: AbbVie Form 10 K
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 13.63 Billion |
| Estimated 2026 Value | USD 15.41 Billion |
| Projected 2034 Value | USD 34.87 Billion |
| CAGR (2026-2034) | 11.04% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Hoffmann-La Roche Ltd., Daiichi Sankyo, Pfizer Inc., Gilead Sciences, AstraZeneca |

to learn more about this report Download Free Sample Report
Antibody Drug Conjugates Market Drivers
Manufacturing Scale-up Drives the Antibody Drug Conjugates Market Growth
The surge in ADC approvals has created supply challenges in the market. To overcome this, in January 2024, Samsung Biologics launched a new ADC facility, and Lonza also acquired Genentech's large-scale biologics manufacturing site in Vacaville in October 2024. These development shows that manufacturers are making production a strategic priority. Thus, manufacturers are scaling up production and securing supply chains to prepare for global demands and reduce the risk of shortage, thereby driving the market growth for ADCs.
Prescribing Adcs in Early Cancer Management Propels the Growth of the ADC Market
Global manufacturers are focusing on replacing chemotherapy with ADCs is a major factor supporting the adoption of ADCs in cancer therapy.
- For example, global manufacturers are designing trials to replace chemotherapy with ADC as a first-line therapy itself. In 2025, DESTINY-Breast09 trial proved that trastuzumab deruxtecan, when combined with pertuzumab, demonstrated higher clinical activity than chemotherapy for treating patients with HER2+ metastatic breast cancer.
Thus, by replacing chemotherapy, ADC manufacturers are developing new standards of care, where ADC is becoming a first-line treatment, which will boost the company’s revenue growth in the long run.
Market Restraints
Pricing and Rebate Competition Restrain the ADC Revenue Generation
Pricing, reimbursement, and competitive pressure reduce the revenue per unit, even when the product volume rises are a major restraint for ADC manufacturers. Gilead’s public filings and quarterly reporting for 2024 call out reimbursement pressures, rebates, and competition as explicit risks that are lowering prices of branded ADCs.
- For instance, the company’s oncology ADC Trodelvy sales increased from USD 1,063 million in 2023 to USD 1,315.0 million in 2024; however, the company finds that payer dynamics and price erosion remain key restraints for future revenue growth of ADC manufacturers.
Market Opportunities
Expanding the Application beyond Breast Cancer is A Key Opportunity for ADC Market Growth
Breast cancer is a major indication for ADC use; manufacturers are focusing on extending it into other solid tumors.
- For example, in June 2025, datopotamab deruxtecan was approved to treat non-small cell lung cancer by the FDA. Thus, for manufacturers, expanding ADC into lung cancer is a major opportunity because of the high number of patients suffering from lung cancer with high medical needs.
Thus, expanding into multiple cancers will help manufacturers to diversify revenue generation and gain a strong foothold in the market.
Regional Analysis
North America Market Trends
North America dominated the antibody drug conjugates market in 2025, accounting for 44.87% market share. This dominance is attributed to strong clinical trial leadership, high uptake of ADCs, and a growing number of antibody approvals in the U.S. Furthermore, strong reimbursement support for high-cost oncology drugs is making ADCs available at low cost, leading to increased consumption. For instance, Trodelvy’s U.S. sales nearly doubled in 2024, showcasing high market uptake. Moreover, the region also benefits from a strong supply chain network, developed clinical infrastructure, and faster adoption of ADCs as compared to other regions.
Asia Pacific Market Growth Factor
Asis Pacific is emerging as the fastest-growing region with a CAGR of 12.7% from 2026-2034, owing to increasing local innovations, strong regulatory support, and expanding patient access. In this region, countries such as China, Japan, and South Korea are developing their own ADCs. For example, in May 2025, RemeGen’s disitamab vedotin became the first China-approved ADC signaling domestic innovations. In addition, the growing cancer incidences and large patient population are also making the Asia Pacific the fastest-growing region. For example, in 2025, Daiichi Sankyo’s Datroway (datopotamab deruxtecan) was approved in Japan to treat the highly prevalent breast cancer.
Source: Straits Analysis
Countries Analysis
U.s. Market Trends
U.S. accounts for the dominant share in the antibody drug conjugates market, driven by the highest number of ADC approvals, the development of novel ADCs through key market players, and a surge in patent application filing for ADCs. The table below highlights the number of ADC patents filed by various countries.
Table: Countries Holding ADC Patent
|
Country |
*No.Of Patents |
|
U.S. |
6741 |
|
Canada |
346 |
|
UK |
543 |
|
Germany |
525 |
|
France |
372 |
|
Italy |
138 |
|
Switzerland |
392 |
|
China |
2445 |
|
Japan |
502 |
|
India |
85 |
|
South Korea |
399 |
|
Israel |
187 |
Source: American Chemical Society
U.S. is the highest ADC patent-holding country, attributed to various factors such as high government investments (NIH Investment USD 47 Billion in 2024), which fuels the novel research that needs intellectual rights. Moreover, FDA’s leadership in approving ADCs like Trodelvy, Enhertu, and Padcev are making a big commercial market, giving manufacturers a strong motive to secure their drug with a patent.
China Market Growth Factors
China antibody drug conjugates market growth is driven by a surge in ADC clinical trials, increasing prevalence of cancers and autoimmune diseases, and accelerated development of domestic ADCs. Furthermore, China is also becoming the largest filer of patents across the globe through companies such as RemeGen, taking the lead with its ADC disitamab vedotin, being among the first approved in China. Moreover, the Chinese government has also prioritized biotech in its national policies, making funding and approvals faster for ADCs.
Germany Market Trends
Germany's market is witnessing significant growth due to strong demand for targeted therapies and rising cancer incidences. Additionally, innovation in linker technologies for non-cleavable linkers, as in Kadcyla, further supports the market growth. Moreover, local manufacturing hubs are expanding ACD pipelines by raising funding for ADC research, thus creating new opportunities for market growth.
- March 2025: Heidelberg Pharma received USD 20 million for ADC development.
- March 2024: Tubulis successfully completes USD 138.8 million series B financing to support clinical evaluation of solid tumor ADC drug.
Switzerland Market Growth Factors
Switzerland’s advancement is driven by Roche’s ADCs, such as Kadcyla, and Lonza, known as one of the largest ADC manufacturing hubs in the country. These factors are collectively driving the ADC market growth in Switzerland. Moreover, growing R&D investment by domestic players to support ADC pipelines and a favorable European regulatory framework drive the market growth for antibody drug conjugates.
Japan Market Trends
Japan is an emerging country in the antibody drug conjugates market, due to strong regulatory support leading to new ADC approvals. For instance, Japanese company Daiichi Sankyo significantly contributes to ADC innovations and currently has 3 candidates in the clinical pipeline. The company is also partnered with AstraZeneca for Enhertu. All the aforementioned factors position Japan as an emerging player in the market.
Product Type Insights
The Kadcyla segment dominated the market with a revenue share of 28.86% in 2025. This growth is attributed to its highest sales in the U.S. and the rest of the world. Additionally, in 2025, Roche expanded global access programs, showcasing Kadcyla in new healthcare systems across the Asia Pacific and Latin America, which led to increased market adoption. Moreover, the expanding application of Kadcyla for HER2-positive early breast cancer further drives the market penetration.

Source: Straits Analysis
Application Insights
The breast cancer segment dominated the market in 2025 and is anticipated to register the fastest CAGR of 12.4% during 2026-2034. This dominance is attributed to the high prevalence of breast cancer worldwide and the increasing number of ADC approvals for breast cancer treatment. Currently, Kadcyla, Trodelvy, and Enhertu are highly revenue-generating ADCs that are primarily approved for the treatment of Breast cancer. Thus, all aforementioned factors support segmental market growth.
Target Insights
The HER2 segment dominated the market in 2025, as it is one of the best biomarkers in oncology treatment. Recently, Enhertu’s approval for HER2-positive targets has built physician trust, making HER2-targeted ADC a first choice in the treatment. Moreover, a high clinical trial success rate compared to other targets such as Trop-2 and CD30 has supported the positive clinical trial results of the HER2 target.
Competitive Landscape
The global antibody drug conjugates market is highly consolidated, with fewer players accounting for the maximum market share. The major key players in the market are Pfizer Inc., F. Hoffmann-La Roche Ltd., AstraZeneca, AbbVie Inc., Gilead Sciences, and others.
These players are actively engaged in various market strategies such as product approval, partnership, acquisition, and product launch to remain competitive in the market.
Remegen: An Emerging Market Player
RemGen is a domestic manufacturer and developer of ADCs in China. The company is actively engaged in novel ADC development through partnerships with global companies. The company is emerging as a significant player in the market through its recent ADC approval.
- In May 2025, RemeGen Co., Ltd. received the approval for disitamab vedotin by China’s National Medical Products Administration (NMPA) to treat HER2-positive advanced breast cancer.
List of Key and Emerging Players in Antibody Drug Conjugates Market
- Hoffmann-La Roche Ltd.
- Daiichi Sankyo
- Pfizer Inc.
- Gilead Sciences
- AstraZeneca
- AbbVie Inc.
- ADC Therapeutics
- Genmab A/S
- Astellas Pharma Inc.
- RemeGen Co., Ltd.
- GSK plc
- Takeda Pharmaceuticals
- Hansoh Pharmaceutical Group Company Limited.
- Eli Lilly and Company
- Bristol-Myers Squibb Company
to learn more about this report Download Market Share
Recent Development
- 23rd June 2025: AstraZeneca’s Datroway has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of EGFR-mutated non-small cell lung cancer. Along with the approval, Datroway has also received Breakthrough Therapy Designation by the FDA. This approval is driving revenue growth of AstraZeneca and Daiichi Sankyo.
- 14th May 2025: The U.S. Food and Drug Administration (FDA) has approved AbbVie’s EMRELIS, a c-Met-directed ADC. Through this approval, AbbVie is commercializing the first and only approved ADC to treat non-squamous non-small cell lung cancer.
Analyst Opinion
As per the analyst's opinion, the ADC market is gaining strong momentum as the pharmaceutical companies push for more precise and effective cancer treatments. The shift from traditional chemotherapy towards targeted therapies is the major factor supporting the ADC adoption for cancer therapies. Moreover, ongoing ADC approvals, with expanding applications in hematology and solid tumors, reflect growing clinical acceptance.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 13.63 Billion |
| Market Size in 2026 | USD 15.41 Billion |
| Market Size in 2034 | USD 34.87 Billion |
| CAGR | 11.04% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Application, By Target |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Antibody Drug Conjugates Market Segments
By Product Type
- Adcetris
- Kadcyla
- Enhertu
- Padcev
- Trodelvy
- Others
By Application
- Blood Cancer
- Breast Cancer
- Urothelial Cancer
- Lung Cancer
- Others
By Target
- CD33
- HER2
- CD30
- TROP2
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































