Aseptic Connectors & Welders Market Size, Share & Trends Analysis Report By Product (Aseptic Connectors, Connection type, Barbed fittings, Luer locks, Genderless, Others, Tubing Size, 1/16 Inch, 1/4 Inch, 3/8 Inch, Aseptic Welders), By Application (Upstream Bioprocessing, Media Preparation, Cell Inoculation, Cell Expansion, Sampling, Other Applications, Downstream Bioprocessing, Purification, Filtration, Fluid Transfer, Harvest & Fill-finish Operations, Product Collection, Product Filling, QC Testing), By End Use (Biopharmaceutical & Pharmaceutical Companies, OEMs, CROs & CMOs, Academic & Research Institutes) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Aseptic Connectors & Welders Market Overview
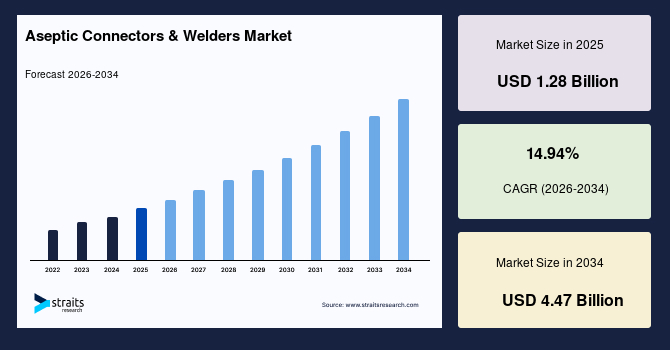
The global aseptic connectors & welders market size is valued at USD 1.28 billion in 2025 and is estimated to reach USD 4.47 billion by 2034, growing at a CAGR of 14.94% during the forecast period. The consistent market growth is supported by the rising adoption of closed system bioprocessing to reduce contamination risk across biologics manufacturing workflows.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 51.14% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 16.94%.
- Based on Product, the aseptic welders segment is anticipated to register the fastest CAGR of 15.23%.
- Based on the Application, the upstream bioprocessing segment dominated the market with 46.78%.
- Based on End Use, the OEMs segment dominated the market with a revenue share of 40.12%.
- The U.S. dominates the aseptic connectors & welders’ market, valued at USD 517.50 million in 2024 and reaching USD 592.58 million in 2025.
Table: U.S. Aseptic Connectors & Welders Market Size (USD Million)
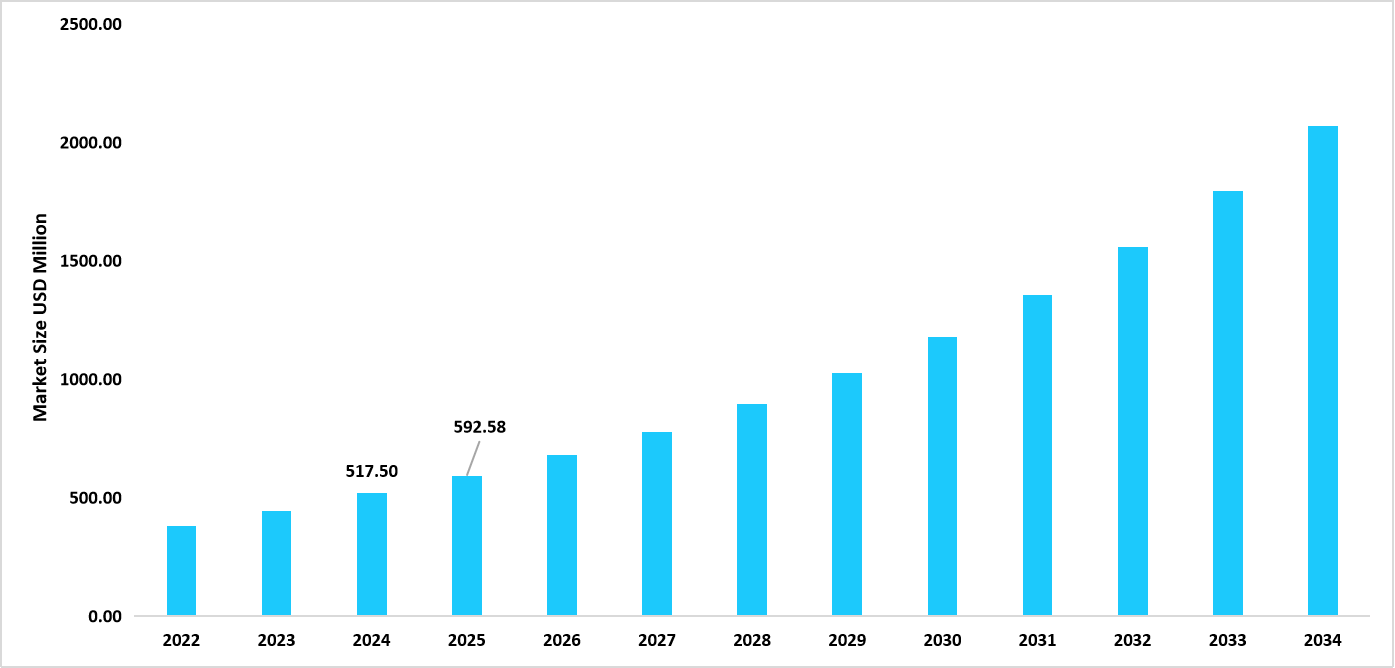
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.28 billion
- 2034 Projected Market Size: USD 4.47 billion
- CAGR (2026-2034): 14.94%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The aseptic connectors and welders market comprises sterile fluid transfer and tubing joining solutions used to maintain contamination-free connections across bioprocessing and pharmaceutical manufacturing workflows. By product, the market includes aseptic connectors and aseptic welders, where aseptic connectors are segmented by connection type, such as barbed fittings, luer locks, genderless connectors, and others, and by tubing size, including 1/16 inch, 1/4 inch, 3/8 inch, and other sizes, while aseptic welders enable sterile joining of thermoplastic tubing without compromising process integrity.
By application, the market spans upstream bioprocessing activities, including media preparation, cell inoculation, cell expansion, sampling, and other applications; downstream bioprocessing functions, such as purification, filtration, sampling, fluid transfer, and other applications; and harvest and fill finish operations encompassing product collection, filtration, product filling, sampling, and QC testing. By end use, aseptic connectors and welders are utilized across biopharmaceutical and pharmaceutical companies, OEMs, CROs and CMOs, and academic and research institutes, supporting closed system processing, process scalability, and regulatory-compliant manufacturing across clinical and commercial production environments.
Latest Market Trends
Shift From Open Fluid Transfer Assemblies To Fully Closed Single-Use Connection Systems
Bioprocessing operations are transitioning from open tubing connections and manual aseptic techniques toward closed system aseptic connectors and welders that maintain sterility throughout fluid transfer steps. Open handling methods rely on cleanroom controls and operator discipline, which introduces exposure risk during media transfer, sampling, and product movement. Closed systems using aseptic connectors and welding technologies enable sterile joining and disconnection without environmental contact, supporting consistent process conditions across upstream, downstream, and fill finish workflows while aligning with stringent contamination control expectations.
Shift From Fixed Stainless Steel Infrastructure To Flexible Single-Use Manufacturing Platforms
Manufacturers are moving away from permanent stainless steel piping toward flexible single-use bioprocessing architectures that rely on disposable tubing assemblies integrated with aseptic connectors and welders. Fixed systems require extensive cleaning, validation, and downtime between batches, whereas single-use configurations support faster changeovers, multiproduct manufacturing, and scale-out production models. Aseptic connection technologies play a central role in enabling modular facility layouts and rapid process reconfiguration.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.28 Billion |
| Estimated 2026 Value | USD 1.46 Billion |
| Projected 2034 Value | USD 4.47 Billion |
| CAGR (2026-2034) | 14.94% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Aseptic Group, L. Gore & Associates, Inc., Meissner Filtration Products, Inc., Terumo Corporation, Watson-Marlow Fluid Technology Solutions |
to learn more about this report Download Free Sample Report
Market Driver
Expansion Of Biologics and Cell Therapy Manufacturing Pipelines
Rising production of monoclonal antibodies, vaccines, and cell-based therapies is increasing demand for sterile fluid handling solutions that support high throughput and regulatory-aligned manufacturing. Aseptic connectors and welders enable contamination-controlled transfer of media, buffers, and drug substances across complex processing stages, reinforcing their adoption in commercial-scale and clinical production facilities.
Market Restraint
Technical Compatibility Limitations Across Tubing Materials and Formats
Variation in tubing polymers, wall thickness, and diameters creates integration challenges for aseptic welding and connector compatibility. Mismatch between system components may restrict flexibility in process design and increase qualification effort, particularly in multi-vendor single-use assemblies.
Market Opportunity
Rising Adoption Of Modular and Decentralized Manufacturing Facilities
Growth of smaller-scale, regional, and modular biomanufacturing sites is creating demand for compact aseptic connectors and welders that support localized production and rapid deployment. These facilities prioritize flexible closed systems that integrate easily with disposable assemblies, opening avenues for tailored connection solutions across diverse manufacturing environments.
Regional Analysis
North America represents a leading region in the aseptic connectors and welders market with a market share of 51.14%, supported by extensive deployment of closed system fluid handling technologies across biopharmaceutical manufacturing and research environments. The region demonstrates widespread integration of aseptic connectors and welding systems within large-scale biologics production facilities, pilot plants, and development laboratories. Mature single-use manufacturing adoption and advanced quality control frameworks support consistent utilization of sterile connection technologies.
In the U.S., market expansion is reinforced by a high concentration of biologics developers and manufacturing sites that integrate aseptic connectors and welders across upstream, downstream, and fill finish workflows to support process continuity and contamination-controlled operations.
Asia Pacific Market Insights
Asia Pacific registers the fastest regional growth of 16.94%, driven by the expansion of biomanufacturing capacity and the increasing establishment of biologics production sites across emerging economies. Facilities in the region are integrating aseptic connectors and welders into new manufacturing lines designed around modular and single-use architectures.
In China, growth is supported by the development of large-scale biologics facilities within industrial parks and hospital-affiliated manufacturing centers, where sterile fluid transfer systems are incorporated across production stages. Rising domestic manufacturing activity and increasing local equipment integration sustain regional momentum.
Regional Market share (%) in 2025
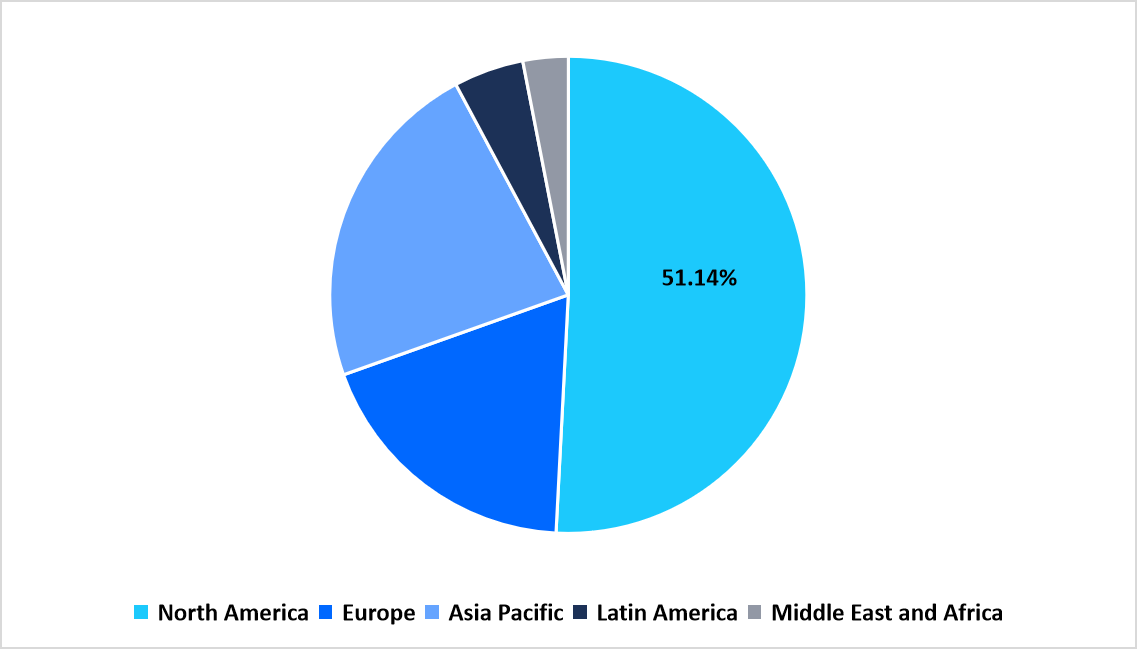
Source: Straits Research
Europe Market Insights
Europe maintains steady market adoption through the incorporation of aseptic connection technologies within pharmaceutical manufacturing networks and academic research facilities. Production sites across the region utilize standardized sterile fluid handling components to support batch consistency and process control.
In Germany, growth is supported by the integration of aseptic connectors and welders within pharmaceutical production facilities and research laboratories focused on biologics development. Alignment between academic research programs and industrial manufacturing supports stable regional uptake.
Latin America Market Insights
Latin America experiences gradual expansion as pharmaceutical manufacturers and research institutions strengthen sterile processing capabilities. Facilities across the region are adopting aseptic connectors and welders to support controlled fluid transfer within production and laboratory settings.
In Brazil, growth is reinforced by the modernization of pharmaceutical manufacturing plants and the expansion of research laboratories, where sterile connection technologies are incorporated into evolving bioprocessing workflows.
Middle East and Africa Market Insights
The Middle East and Africa region advances through the development of pharmaceutical manufacturing infrastructure and research capabilities. Adoption of aseptic connectors and welders supports sterile processing within specialized healthcare manufacturing units and research laboratories.
In the United Arab Emirates, market momentum is supported by the deployment of advanced biomanufacturing facilities and research centers that integrate closed system fluid handling technologies, strengthening the country’s regional position.
Product Insights
Aseptic connectors represent the dominating product category, supported by their extensive use across closed system fluid transfer operations in bioprocessing environments. These connectors are widely adopted across multiple connection types and tubing sizes to support sterile transfer of media, buffers, and process fluids across varied manufacturing stages. Their adaptability across single-use assemblies and compatibility with modular production setups reinforce sustained utilization within both development and commercial-scale operations.
Aseptic welders are anticipated to register the fastest growth at 15.23%, driven by increasing deployment in continuous processing and multiproduct facilities. Welding systems support sterile tubing joining without additional components, which aligns with streamlined process design and reduced consumable dependency across dynamic manufacturing workflows.
Application Insights
Upstream bioprocessing dominated the application segment with a revenue share of 46.78%, reflecting extensive use of aseptic connectors and welders during media preparation, cell inoculation, expansion, and sampling activities. These stages require repeated sterile connections to maintain controlled environments during the early phase of biological production, which sustains strong demand within upstream operations.
Downstream bioprocessing is projected to grow at the fastest rate with a CAGR of 15.68%, supported by the increasing complexity of purification, filtration, and fluid transfer steps in biologics manufacturing. Sterile connection technologies play a central role in maintaining process continuity during high-value product handling and final purification stages.
End Use Insights
OEMs accounted for the dominating end-use segment with a share of 40.12%, supported by their role in integrating aseptic connectors and welders into single-use assemblies, equipment platforms, and turnkey bioprocessing solutions supplied to manufacturers.
CROs and CMOs are expected to witness the fastest growth at 15.71%, driven by rising outsourcing of biologics production and clinical manufacturing, where flexible sterile connection technologies support diverse client requirements and rapid project transitions.
End Use Market share (%) in 2025
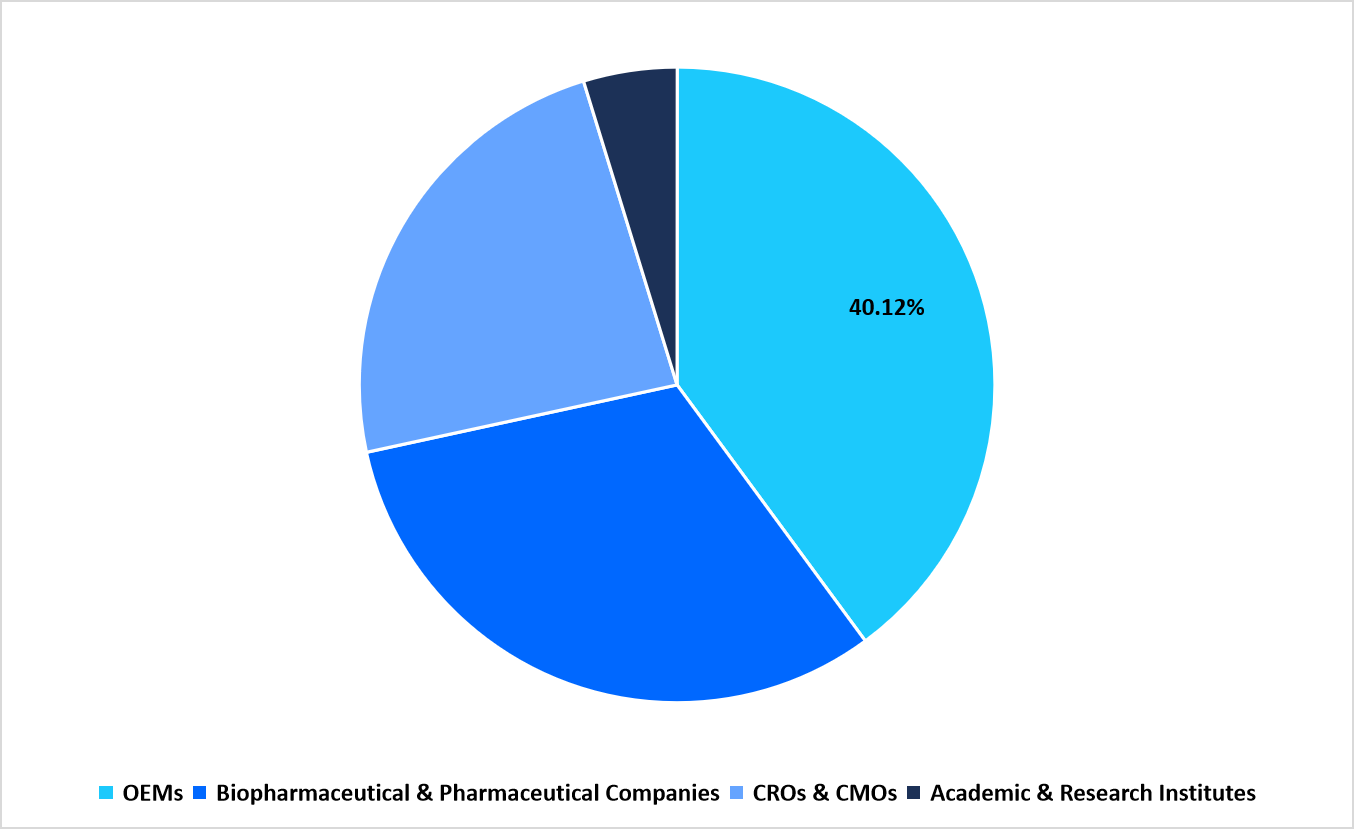
Source: Straits Research
Competitive Landscape
The global aseptic connectors and welders market is moderately fragmented, with established bioprocess equipment suppliers and specialized sterile fluid handling manufacturers operating across biopharmaceutical manufacturing, contract production, and research environments. Market participants compete through breadth of product portfolios, integration of connectors and welders into single-use assemblies, and alignment with evolving bioprocessing workflows across upstream, downstream, and fill finish operations. Companies focus on compatibility with diverse tubing formats, scalability across development and commercial production, and integration within modular manufacturing platforms to strengthen their market positioning.
Pall Corporation: An Emerging Market Player
Pall Corporation holds a strong position in the aseptic connectors and welders market through its range of sterile connection solutions designed for closed system fluid transfer in bioprocessing environments. The company supplies aseptic connectors and welding-compatible tubing assemblies that support media transfer, sampling, and product movement across biologics manufacturing workflows. Its offerings are widely used within single-use processing platforms deployed by biopharmaceutical companies and contract manufacturers. Pall continues to expand its market presence by aligning sterile connection technologies with the increasing adoption of disposable bioprocess systems and flexible manufacturing configurations.
List of Key and Emerging Players in Aseptic Connectors & Welders Market
- Aseptic Group
- L. Gore & Associates, Inc.
- Meissner Filtration Products, Inc.
- Terumo Corporation
- Watson-Marlow Fluid Technology Solutions
- Pall Corporation
- Sartorius AG
- Cytiva
- Saint-Gobain
- Parker Hannifin Corporation
- CPC
- Nordson Corporation
- Others
Strategic Initiatives
- January 2025: CPC (Colder Products Company) launched the MicroCNX Nano Series aseptic connectors in the U.S. at the Advanced Therapies Week meeting in Dallas.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.28 Billion |
| Market Size in 2026 | USD 1.46 Billion |
| Market Size in 2034 | USD 4.47 Billion |
| CAGR | 14.94% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Aseptic Connectors & Welders Market Segments
By Product
- Aseptic Connectors
- Connection type
- Barbed fittings
- Luer locks
- Genderless
- Others
- Tubing Size
- 1/16 Inch
- 1/4 Inch
- 3/8 Inch
- Aseptic Welders
By Application
- Upstream Bioprocessing
- Media Preparation
- Cell Inoculation
- Cell Expansion
- Sampling
- Other Applications
- Downstream Bioprocessing
- Purification
- Filtration
- Fluid Transfer
- Harvest & Fill-finish Operations
- Product Collection
- Product Filling
- QC Testing
By End Use
- Biopharmaceutical & Pharmaceutical Companies
- OEMs
- CROs & CMOs
- Academic & Research Institutes
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































