ATP Assays Market Size, Share & Trends Analysis Report By Type (Luminometric ATP Assays, Enzymatic ATP Assays, Bioluminescence Resonance Energy Transfer (BRET) ATP Assays, Cell-based ATP Assays, Others), By Application (Clinical Diagnostics, Drug Discovery and Development, Others), By End User (Pharmaceutical and Biotechnology Companies, Clinical Diagnostics Laboratories, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
ATP Assays Market Overview
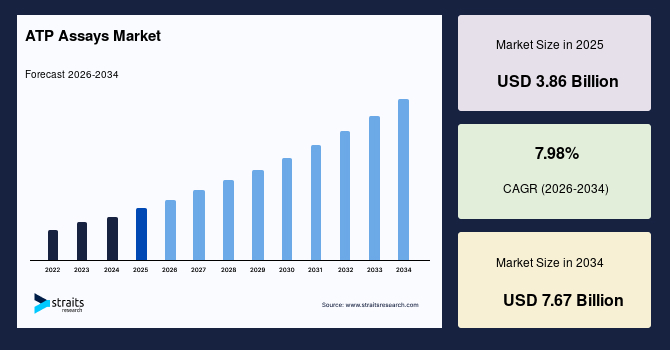
The global ATP assays market size is estimated at USD 3.86 billion in 2025 and is projected to reach USD 7.67 billion by 2034, growing at a CAGR of 7.98% during the forecast period. Strong growth of the market is propelled by the rising incorporation of ATP-based luminescence tools in high-throughput drug screening, growing preference for energy metabolism analysis in advanced cell models, and expanding use of ATP quantification in microbial monitoring across clinical, industrial, and environmental applications.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 40.13% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 9.98%.
- Based on Type, Luminometric ATP assays dominated the market with a revenue share of 35.46%.
- Based on Application, Drug Discovery and Development dominated the market in 2025 with a revenue share of 34.56%.
- Based on End User, Pharmaceutical and Biotechnology Companies dominated the market in 2025 with 41.23% market share.
- The U.S. dominates the global ATP assays market, valued at USD 1.30 billion in 2024 and reaching USD 1.40 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
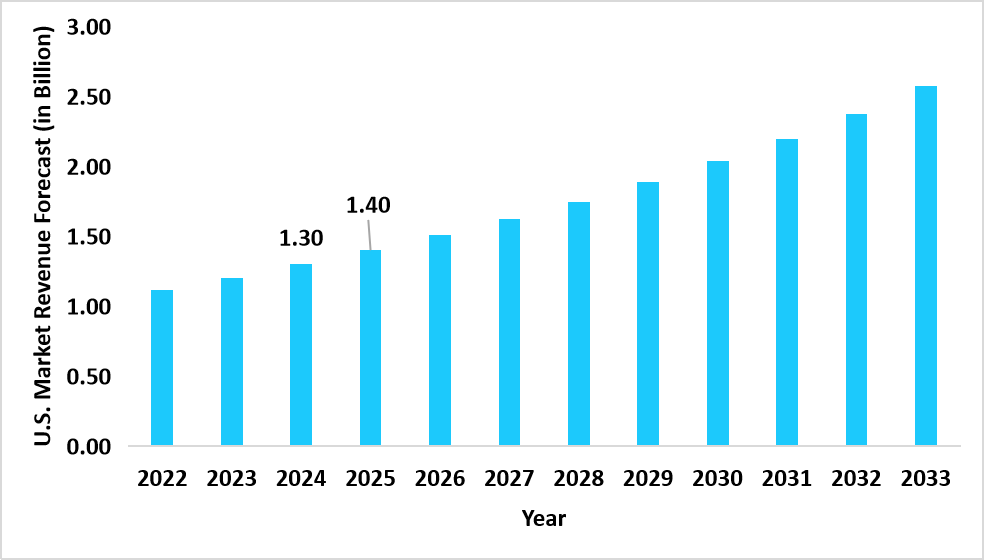
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 3.86 billion
- 2034 Projected Market Size: USD 7.67 billion
- CAGR (2025 to 2034): 7.98%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The ATP assays market comprises a range of analytical tools designed to quantify adenosine triphosphate (ATP) as an indicator of cellular energy levels, viability, and metabolic activity across research, clinical, and industrial settings. These assays include luminometric ATP assays, enzymatic ATP assays, BRET-based ATP detection formats, cell-based ATP measurement kits, and other specialized ATP quantification methods. They are widely applied in clinical diagnostics, drug discovery, and additional biological testing workflows where ATP serves as a central biomarker for cellular function. Key end users include pharmaceutical and biotechnology companies, clinical diagnostics laboratories, and various research institutions that rely on ATP assays to evaluate cytotoxicity, monitor microbial contamination, analyze metabolic pathways, and support screening programs in both manual and automated laboratory environments.
Latest Market Trends
Rising Integration of ATP Profiling in Organoid and 3D Culture Systems
A growing trend emerges as research groups expand the use of ATP-based viability measurements within organoids and 3D culture models. These systems require metabolic tracking methods that can capture variations in energy output across layered cellular structures. ATP-based readouts are increasingly used to evaluate maturation, structural integrity, and response to compound exposure in physiologically complex models. This trend is gaining traction among laboratories exploring disease modeling and advanced cell-based platforms.
Adoption of Ultra-High Sensitivity ATP Platforms for Micro-Scale Sampling
The major trend is the rise of ATP assays designed for very small sample volumes used in microfluidic devices and miniature culture chambers. As researchers shift toward low-volume experimentation, ATP detection tools tailored for micro-scale reactions are becoming more common. These platforms support studies involving rare cells, niche microbial communities, and specialized cellular microenvironments, creating new pathways for innovation in small-format testing.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 3.86 Billion |
| Estimated 2026 Value | USD 4.15 Billion |
| Projected 2034 Value | USD 7.67 Billion |
| CAGR (2026-2034) | 7.98% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | BioVision, Inc., 3M, Reaction Biology, Thermo Fisher Scientific Inc., Promega Corporation |
to learn more about this report Download Free Sample Report
ATP Assays Market Driver
Expansion of Energy Metabolism Research Across Multi-Disciplinary Fields
A key driver arises from wider interest in ATP dynamics across fields such as neurobiology, immunometabolism, environmental microbiology, and bio-production science. Researchers increasingly use ATP quantification to map cellular energy fluctuations, evaluate stress responses, and study metabolic shifts caused by environmental or chemical stimuli. This broadening research scope supports steady integration of ATP-based tools into diverse scientific programs.
Market Restraint
Constraints Linked to ATP Instability During Prolonged Sample Handling
A restraint in the market stems from challenges associated with ATP degradation when samples are stored or processed for extended periods. Variability caused by time-sensitive ATP loss reduces assay accuracy if strict handling steps are not followed. Laboratories working with field samples or delayed processing schedules face added complexity, which slows wider adoption in settings where rapid preparation is difficult.
Market Opportunity
Growth of Automated ATP Platforms for Distributed Laboratory Networks
A strong opportunity arises from developing automated ATP testing systems that can be deployed across decentralized laboratory clusters, biotechnology parks, and academic research networks. These platforms can streamline assay preparation, reduce manual steps, and support unified data-sharing systems across multiple sites. Adoption of automated ATP workflows creates new prospects for coordinated research programs and multi-center screening studies.
Regional Analysis
North America holds a leading position in the ATP assays market with a market share of 40.13% due to high adoption of advanced cell-based research tools and strong demand from pharmaceutical and biotechnology companies conducting screening studies. The region sees continuous expansion in academic laboratories that use ATP-based luminescence assays for metabolic studies, toxicity evaluations, and compound profiling. Growth is shaped by increased automation in research facilities, broad availability of assay kits through direct and online channels, and rising interest in high-throughput platforms for drug development projects. Manufacturers continue to introduce upgraded ATP detection chemistries that support longer signal stability and simplified workflows, encouraging wider uptake across research environments.
The U.S. market grows as drug developers, CROs, and university laboratories integrate ATP assays into cell viability studies, energy metabolism research, and microbial contamination checks. Adoption increases through the expansion of screening programs in oncology, immunology, and metabolic disease research. Assay kits gain traction through strong distribution networks, e-commerce channels, and procurement partnerships with research institutes. Local presence of leading assay manufacturers accelerates the availability of new detection formats that support both manual and automated instruments across laboratory settings.
Asia Pacific Market Insights
Asia Pacific registers the fastest growth of 9.98% driven by the increasing establishment of biotechnology start-ups, greater investment in life science education, and expanding research capacity in academic institutes. Growing interest in cell-based screening, microbial monitoring, and ATP-mediated viability analysis contributes to rising assay adoption across laboratories. Availability of affordable assay kits through regional distributors strengthens market reach, while digital procurement platforms improve accessibility for smaller research groups. Government-funded programs supporting drug discovery stimulate the incorporation of ATP-based assays into early-stage research activities.
India’s market expands as universities, pharmaceutical manufacturers, and diagnostic laboratories incorporate ATP assays for viability checks, cytotoxicity measurements, and microbial detection. Growth is influenced by the expansion of biotech parks, the rising number of funded research projects, and increased awareness of luminescence-based assays among laboratory scientists. Procurement through online scientific marketplaces broadens accessibility of assay kits from global brands. Growing participation in international research collaborations further increases assay adoption across both public and private facilities.
Pie Chart: Regional Market Share, 2025
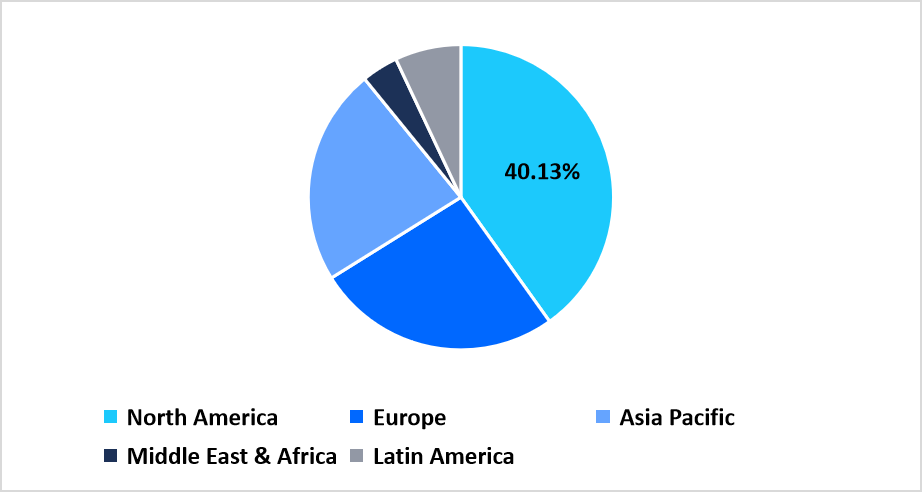
Source: Straits Research
Europe Market Insights
Europe advances with continued investment in biomedical research infrastructure and rising uptake of ATP-based cell viability platforms in toxicology screening, metabolic profiling, and microbial quantification. Laboratories across the region integrate luminescent assays to streamline workflows in drug discovery, environmental testing, and food safety analysis. Demand rises through the expansion of collaborative research projects and the wide availability of multi-format ATP detection kits through scientific distributors. Growing emphasis on standardization of assay protocols supports increased use across regulated laboratory environments.
Germany’s market progresses due to the strong involvement of research universities, biotechnology firms, and industrial laboratories that use ATP assays for quality checks, metabolic studies, and high-throughput viability screening. Structured R&D programs in pharmaceuticals and chemicals drive consistent adoption of luminescent detection formats. Broad availability of assay kits through laboratory suppliers and digital procurement portals supports continuous test usage. Engagement in EU-funded research networks contributes to rising ATP assay consumption across varied application areas.
Middle East and Africa Market Insights
The Middle East and Africa region moves forward with the growing establishment of research laboratories, university life science programs, and industrial testing facilities adopting ATP-based assays for microbial evaluation, cell-based studies, and bio-production monitoring. Expansion of cold-chain logistics improves access to imported assay kits across urban scientific hubs. Rising participation in biotechnology training programs and laboratory modernization initiatives encourages uptake of luminescence-based ATP detection across clinical and industrial settings.
The UAE market develops as research centers, private laboratories, and biotechnology firms integrate ATP assays into cell health evaluation, sterility assessments, and metabolic studies. Growth is strengthened by expanding laboratory infrastructure in Dubai and Abu Dhabi, which increases demand for luminescent viability assays. The presence of global distributors improves access to diverse detection kits and reagents. Introduction of advanced R&D initiatives across life science parks contributes to rising assay consumption across both academic and commercial laboratories.
Latin America Market Insights
Latin America depicted upward movement as universities, pharmaceutical companies, and food processing industries adopt ATP assays for microbial screening, cytotoxicity testing, and cell health evaluation. Expansion of laboratory training programs enhances knowledge of luminescent assays among early-career researchers. Online scientific retailers improve accessibility to assay kits, while regional distributors increase supply across major metropolitan areas. Growing interest in modern cell culture practices supports the wider application of ATP-based assays.
Brazil’s market rises as research institutes, biotechnology firms, and industrial laboratories use ATP assays for contamination monitoring, viability studies, and compound screening. Increased funding in public research centers and expansions in private laboratory networks stimulate assay adoption. Distribution partnerships with international assay manufacturers improve the availability of luminescence kits in academic and industrial clusters. Training initiatives within universities support growing familiarity with ATP-based detection methods across laboratory personnel.
Type Insights
Luminometric ATP assays dominated the market with 35.46%, driven by their widespread use in cell viability studies, metabolic evaluations, and microbial screening across research and industrial laboratories. The segment gains strength from high adoption of luminescence-based detection formats that offer clear signals, straightforward workflows, and compatibility with automated screening platforms. Continuous expansion of screening programs in academic and commercial labs keeps this category in a leading position.
Enzymatic ATP assays are the fastest-growing segment with 8.12%, supported by rising interest in enzymatic conversions that allow deeper insight into biochemical pathways and ATP turnover. Adoption increases as researchers pursue detailed analysis of enzyme activity, cellular respiration, and pathway-specific reactions. The segment advances further through higher demand for assays suited for kinetic studies and quantitative measurements in specialized research projects.
Application Insights
Drug discovery and development dominated the market with 34.56%, as ATP assays are widely used for cytotoxicity screening, compound selection, and high-throughput viability measurements. The segment benefits from expanding pipelines in oncology, metabolic disorders, and infectious disease research. Large volumes of assay plates processed during lead optimization continue to drive strong uptake of ATP-based luminescent tools across pharmaceutical and biotechnology research facilities.
Clinical diagnostics is the fastest-growing segment with 8.34%, propelled by rising interest in ATP-based microbial detection for sterility checks, infection screening, and sample cleanliness verification. Clinical laboratories adopt these assays for quick ATP quantification that supports routine hygiene monitoring, contamination analysis, and select diagnostic workflows. Increasing incorporation of ATP tests into quality assurance programs drives steady momentum in this category.
End User Insights
Pharmaceutical and biotechnology companies dominated the market with 41.23%, driven by the extensive use of ATP assays across early discovery, lead optimization, and routine viability screening. These organizations rely on ATP-based luminescence kits to evaluate compound potency, monitor metabolic activity, and support high-throughput workflows. Large R&D investments and consistent use of cell-based models maintain this segment’s leading position.
Clinical diagnostics laboratories are the fastest-growing segment with 8.98%, supported by wider adoption of ATP quantification for sanitation checks, contamination investigations, and microbial detection across healthcare settings. The increasing introduction of ATP testing within quality monitoring programs across laboratories and healthcare facilities contributes to greater uptake in this category.
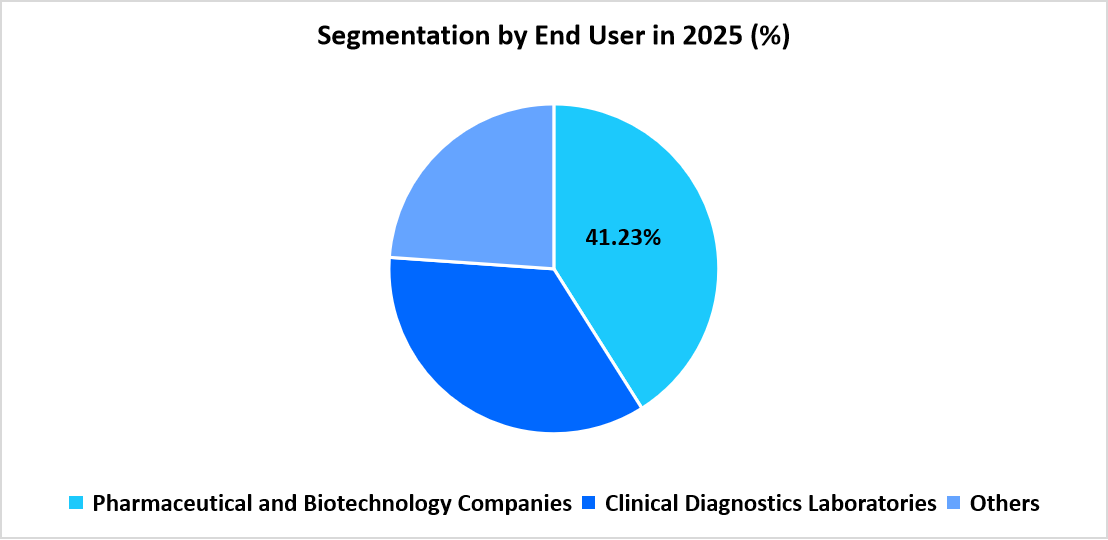
Source: Straits Research
Competitive Landscape
The ATP assays market is moderately fragmented, characterized by a mix of well-established life science companies and an increasing number of niche assay developers specializing in cell viability testing, microbial detection, high-throughput screening, and biochemical analysis.
Biotium: An emerging market player
Biotium, known for its advanced fluorescent and luminescent assay technologies, is rapidly gaining recognition as an innovative entrant in the ATP assays landscape. With products designed for high-throughput screening and extended signal stability, the company delivers solutions that go beyond traditional ATP viability assays by enabling greater workflow efficiency and affordability. Its focus on developing highly sensitive, automation-friendly kits positions Biotium as a strong emerging player contributing to the evolving dynamics of the ATP assays market.
List of Key and Emerging Players in ATP Assays Market
- BioVision, Inc.
- 3M
- Reaction Biology
- Thermo Fisher Scientific Inc.
- Promega Corporation
- Merck KGaA
- Agilent
- Abcam Limited
- Lonza
- DH Life Sciences, LLC
- Hygiena LLC
- BioThema
- Biotium
- AAT Bioquest, Inc
- Abnova Corporation
- Cell Signaling Technology, Inc.
- Others
Strategic Initiatives
- March 2025: Biotium announced the launch of its Steady-ATP HTS Viability Assay Kit, a luminescent assay designed for high-throughput screening (HTS) applications. The assay offered exceptional sensitivity, detecting as few as 16 cells per well in a 384-well plate, and provided a stable luminescent signal with a half-life exceeding 5 hours. Its single-step, homogeneous assay format simplified workflows, making it particularly suitable for automated HTS platforms. By delivering performance comparable to existing assays, such as CellTiter-Glo, at a more affordable price point, the Steady-ATP HTS Viability Assay Kit was positioned to accelerate growth in the ATP assays industry by enhancing accessibility and efficiency in cell viability assessments across pharmaceutical, biotechnology, and academic research settings.
- April 2024: Reaction Biology, a leading provider of drug discovery and development services, announced the launch of its HotSpot ATP-Max KinomeScreen assay platform at the American Association for Cancer Research (AACR) Annual Meeting 2024. This innovative assay enabled kinase profiling at physiologically relevant ATP concentrations (1 mM) across the industry's largest portfolio of kinase targets, utilizing a gold-standard filter binding radiometric assay.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 3.86 Billion |
| Market Size in 2026 | USD 4.15 Billion |
| Market Size in 2034 | USD 7.67 Billion |
| CAGR | 7.98% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
ATP Assays Market Segments
By Type
- Luminometric ATP Assays
- Enzymatic ATP Assays
- Bioluminescence Resonance Energy Transfer (BRET) ATP Assays
- Cell-based ATP Assays
- Others
By Application
- Clinical Diagnostics
- Drug Discovery and Development
- Others
By End User
- Pharmaceutical and Biotechnology Companies
- Clinical Diagnostics Laboratories
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































