Bioprocess Analyzers Market Size, Share & Trends Analysis Report By Product (Instruments, Consumables & accessories), By Analysis Type (Substrate analysis, Metabolite analysis, Concentration detection), By Application (Antibiotics, Recombinant proteins, Biosimilars, Other applications), By End Use (Biopharmaceutical companies, CROs & CMOs, Research & academic institutes, Other End Use) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Bioprocess Analyzers Market Overview
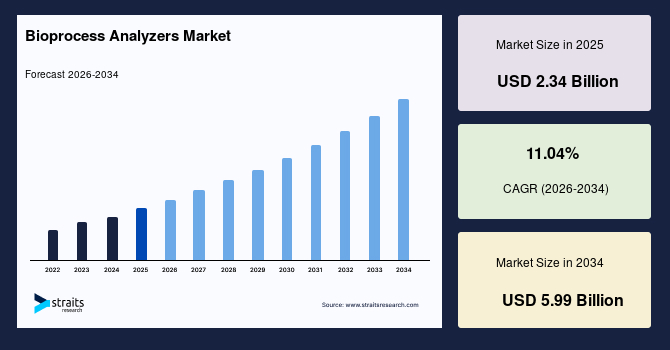
The global bioprocess analyzers market size is valued at USD 2.34 billion in 2025 and is estimated to reach USD 5.99 billion by 2034, growing at a CAGR of 11.04% during the forecast period. The consistent market growth is supported by the rising transition toward data-driven bioprocess environments that rely on continuous measurement to strengthen decision-making across upstream and downstream operations.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 42.24% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 13.04%.
- Based on product, the instruments segment is anticipated to register the fastest CAGR of 12.23% during the forecast period.
- Based on the analysis type, the concentration detection segment held a share of 39.23% in 2025.
- By application, the antibiotics segment held a share of 31.23% in 2025.
- By end use, the biopharmaceutical companies segment held a share of 47.20% in 2025.
- S. dominates the bioprocess analyzers market, valued at USD 809.07 million in 2024 and reaching USD 894.92 million in 2025.
Table: U.S. Bioprocess Analyzers Market Size (USD Million)
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.34 billion
- 2034 Projected Market Size: USD 5.99 billion
- CAGR (2026-2034): 11.04%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The bioprocess analyzers market encompasses instruments and consumables used to measure, track, and interpret critical parameters within fermentation and cell culture workflows across biopharmaceutical production and advanced research environments. These analyzers support substrate analysis, metabolite assessment, and concentration detection, providing continuous insight into nutrient consumption, metabolic behavior, and product formation. Their application spans antibiotic manufacturing, recombinant protein development, biosimilar production, and a wide set of process-driven studies that require structured monitoring for yield optimization and quality assurance. End users include biopharmaceutical companies, CROs, and CMOs that manage outsourced production activities, and research and academic institutes focused on experimental bioprocess design, making analyzers central to controlled, data-driven decision making throughout upstream and downstream operations.
Latest Market Trends
Shift from isolated batch checks to a continuous process linked to analytical evaluation
A major trend in the bioprocess analyzers market is the shift from isolated batch checks to continuous process-linked analytical evaluation. Earlier workflows relied on periodic sampling, which captured only a fraction of the process behavior, resulting in gaps in understanding nutrient flow, metabolic transitions, and culture state changes. Movement toward continuous evaluation allows each stage of the bioprocess to be monitored through uninterrupted data capture. This transition supports more controlled adjustments, enhances visibility into subtle process transitions, and promotes smoother alignment between measurement outputs and production requirements across diverse biologics pipelines.
Shift from manual parameter tracking to automated interpretive data ecosystems
The notable trend is the shift from manual parameter tracking to automated interpretive data ecosystems. Traditional monitoring required extensive manual recording with limited integration between analyzer outputs and process management tools. The adoption of automated interpretive ecosystems connects analyzer readings with centralized data environments that generate structured insights for decision making. This shift improves operational pacing, supports pattern recognition during scale up, and increases the value of measurement streams through coordinated data relationships that guide process refinement.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.34 Billion |
| Estimated 2026 Value | USD 2.59 Billion |
| Projected 2034 Value | USD 5.99 Billion |
| CAGR (2026-2034) | 11.04% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific Inc., Sartorius AG, Nova Biomedical, HoffmannâLa Roche Ltd., MJH Life Sciences |
to learn more about this report Download Free Sample Report
Bioprocess Analyzers Market Driver
Rising pursuit of precision-centered bioprocess control across biologics development
A strong driver for the market is the rising pursuit of precision-centered bioprocess control across biologics development. Producers seek greater clarity in nutrient dynamics, metabolic activity, and concentration profiles throughout cultivation cycles. This pursuit elevates demand for analyzers that track real-time conditions and reinforce structured control strategies across fermentation and cell culture stages. As biological products diversify, the push for precise measurement frameworks strengthens the role of analyzers in guiding consistent production outcomes.
Market Restraint
Variability in measurement outputs when analyzers operate across shifting cultural states
A restraint arises from variability in measurement behavior when analyzers operate across changing cultural states. Fluctuations in cell density, metabolite distribution, and nutrient availability influence sensor performance and require added calibration efforts. These inconsistencies extend processing timelines and call for repeated verification steps to confirm accuracy across evolving conditions. Such variability introduces added layers of planning for groups that target unified data sets across extended production windows.
Market Opportunity
Expansion of digital integration programs that align analyzers with coordinated bioprocess platforms
A broad opportunity is emerging through the expansion of digital integration programs that align analyzers with coordinated bioprocess platforms. Research centers and commercial manufacturers are investing in unified digital environments that connect measurement units with control software, data lakes, and simulation models. This integration strengthens analytical depth, improves flow between measurement points, and supports broader use of predictive strategies during culture optimization. As these programs mature across global production networks, adoption of analyzers is expected to widen through improved compatibility with next-generation bioprocess systems.
Regional Analysis
North America maintains a leading share of 42.24% in the bioprocess analyzers market due to the strong integration of analytical systems across commercial biomanufacturing and high-output research facilities. Growth is driven by structured programs linking federal research divisions with large biopharmaceutical companies, which accelerate the use of analyzer platforms for substrate monitoring, metabolite trending, and concentration assessment in fermentation and cell culture environments. The region benefits from high adoption of digital data pipelines that streamline analyzer outputs into large-scale process control systems across biologics and vaccine production units.
In the U.S., expansion is supported by the rapid incorporation of automated measurement modules within upstream processing suites. National biomanufacturing hubs invest in inline and at-line analyzers that strengthen consistency in monoclonal antibody production, recombinant protein development, and biosimilar scale-up activities.
Asia Pacific Market Insights
Asia Pacific records the fastest growth of 13.04% driven by active development of regional biomanufacturing corridors and specialized training centers focused on advanced process monitoring technologies. Government-funded biotechnology programs support wider access to analytical instruments used for real-time assessment of fermentation runs and cell culture optimization studies. Growing investment in academic laboratories encourages the adoption of analyzers for instructional courses in bioprocess control and metabolic profiling.
In China, demand rises as national initiatives target high throughput biologics manufacturing. Local research parks deploy analyzer units designed for continuous nutrient measurement and cell health assessment, reinforcing the region’s role in large volume production of vaccines and therapeutic proteins.
Regional Market share (%) in 2025
Source: Straits Analysis
Europe Market Insights
Europe records steady growth supported by a regulatory environment that encourages the adoption of structured process analytical technology frameworks across biologics manufacturing facilities. The region advances through coordinated projects that promote harmonized use of bioprocess analyzers for upstream and downstream workflows, ranging from substrate tracking to purification quality checks. European institutes utilize refined sensor-based systems to study cell performance across extended cultivation periods.
In the United Kingdom, domestic growth is linked to partnerships between biotechnology organizations and academic laboratories focused on building advanced fermentation clusters. Research teams integrate analyzer systems for continuous monitoring of critical parameters, which improves consistency in pilot scale biologics development.
Latin America Market Insights
Latin America progresses through the expansion of regional bioprocessing programs that introduce analytical tools into universities and mid scale production facilities. Access to compact analyzer units supports adoption across training laboratories that teach fermentation control, nutrient regulation, and cell performance assessment. Local contract research centers begin incorporating bioprocess analyzers to improve measurement accuracy during early-stage biologics development.
In Brazil, expansion is supported by public investments that strengthen national biopharmaceutical manufacturing capacity. Research parks adopt analyzer instruments for monitoring metabolic patterns across microbial and mammalian cultures, reinforcing the country’s role in regional vaccine and biosimilar production.
Middle East and Africa Market Insights
The Middle East and Africa region advances as institutional procurement programs widen access to bioprocess monitoring instruments in academic and clinical research settings. Shared laboratory networks create opportunities for students and researchers to work with analyzer platforms designed for nutrient evaluation, metabolite tracking, and concentration estimation in small-scale studies. Adoption rises as universities incorporate structured bioprocessing courses that train personnel in analytical measurement principles.
In the United Arab Emirates, growth is driven by the formation of biotechnology clusters where private and academic groups collaborate on biologics development. Local facilities integrate analyzer systems into controlled fermentation units used for feasibility studies, pilot batches, and early formulation research.
Product Insights
The consumables and accessories segment dominated the market with the largest share as routine replacement parts, assay kits, and measurement reagents remain central to continuous analyzer use across fermentation and cell culture units. Consistent demand from commercial biologics production reinforces the leadership of this category within the product landscape.
The instruments segment recorded the fastest growth at 12.23%, driven by wider adoption of integrated measurement units that support structured process evaluation across upstream workflows.
Analysis Type Insights
The concentration detection segment dominated the category with 39.23%, supported by extensive use in quantifying critical process parameters that guide nutrient balance, metabolite control, and batch consistency across bioprocess stages. Its central role in establishing measurement standards strengthens its market position.
The metabolite analysis segment expanded at the fastest pace with 12.45%, propelled by rising interest in detailed metabolic profiling used to refine cell performance and optimize culture conditions across research and production environments.
Application Insights
The antibiotics segment held the dominant share at 31.23%, as analyzer platforms are actively used to assess nutrient consumption, substrate change, and real-time culture behavior during antibiotic fermentation runs. Its broad utilization across industrial production systems sustains its leading status within the application landscape.
The recombinant protein segment achieved the fastest growth with 12.67%, driven by increased deployment of analyzers to support precision monitoring during recombinant expression cycles and controlled scale up procedures.
End Use Insights
Biopharmaceutical companies dominated the market with 47.20%, supported by the wide use of analyzers in process development, optimization of cell culture strategies, and routine quality assessment across commercial biologics pipelines. Their large-scale operations reinforce substantial utilization of analytical platforms.
CROs and CMOs recorded the fastest growth at 12.83%, driven by expanded outsourcing of bioprocess development activities that require frequent measurement of critical attributes for project-based production and testing workflows.
Source: Straits Research Analysis
Competitive Landscape
The global bioprocess analyzers market remains moderately fragmented, with bioprocess instrument manufacturers, analytical technology developers, and biomanufacturing solution providers expanding their presence across upstream and downstream processing workflows. Companies strengthen their position through product refinements, strategic collaborations with biopharmaceutical producers, contract service organizations, and academic laboratories, along with expanding sales channels that support wider deployment across applications such as antibiotics production, recombinant protein development, biosimilar manufacturing, and concentration monitoring.
Sartorius AG: An emerging market player
Sartorius AG advances its presence in the market through analyzers integrated with its broader bioprocessing ecosystem. Its instruments support real-time assessment of substrates, metabolites, and concentration parameters, enabling smooth incorporation into upstream development workflows for biopharmaceutical manufacturers.
List of Key and Emerging Players in Bioprocess Analyzers Market
- Thermo Fisher Scientific Inc.
- Sartorius AG
- Nova Biomedical
- Hoffmann‑La Roche Ltd.
- MJH Life Sciences
- SYSBIOTECH GmbH
- Groton Biosystems
- Randox Laboratories Ltd.
- Agilent Technologies
- MettlerToledo
- Hamilton Company
- Pall Corporation
- Eppendorf SE
- Cytiva
- Waters Corporation
- Others
Strategic Initiatives
- June 2025: Waters Corporation debuted BioResolve Protein A columns with MaxPeak technology offering seven-fold sensitivity gains for antibody titer assays.
- April 2025: Thermo Fisher Scientific introduced the 5 L DynaDrive single-use bioreactor, reporting a 27% productivity boost versus legacy glass systems.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.34 Billion |
| Market Size in 2026 | USD 2.59 Billion |
| Market Size in 2034 | USD 5.99 Billion |
| CAGR | 11.04% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Analysis Type, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Bioprocess Analyzers Market Segments
By Product
- Instruments
- Consumables & accessories
By Analysis Type
- Substrate analysis
- Metabolite analysis
- Concentration detection
By Application
- Antibiotics
- Recombinant proteins
- Biosimilars
- Other applications
By End Use
- Biopharmaceutical companies
- CROs & CMOs
- Research & academic institutes
- Other End Use
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































