Biopharmaceutical Market Size
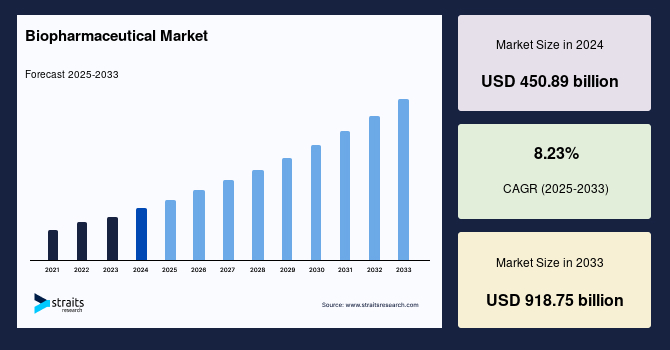
The global biopharmaceutical market size was valued at USD 450.89 billion in 2024 and is estimated to grow from USD 487.99 billion in 2025 to reach USD 918.75 billion by 2033, growing at a CAGR of 8.23% during the forecast period (2025–2033).
The global biopharmaceutical market is primarily driven by the surging global aging population, which has led to a higher demand for advanced biologic therapies to manage age-related diseases and conditions. Additionally, favorable regulatory frameworks across major markets have accelerated the approval process for innovative biopharmaceutical products, encouraging companies to invest more in research and development.
Significant investments from both public institutions and private enterprises have fueled continuous advancements in bioprocessing and biomanufacturing technologies, improving production efficiency and product quality. Furthermore, expanding healthcare infrastructure in emerging economies is creating new opportunities for market growth by increasing patient access to biologics. These combined factors are propelling the biopharmaceutical sector toward rapid expansion worldwide.
Current Market Trends
Integration of Ai and Big Data in Drug Discovery and Clinical Trials
A key trend reshaping the biopharmaceutical market is the integration of artificial intelligence (AI) and big data into drug discovery and clinical trials. AI tools are accelerating early-stage research by identifying promising drug candidates, predicting molecular interactions, and optimizing clinical trial protocols.
- For instance, Alphabet’s Isomorphic Labs raised $600 million to advance AI-driven drug discovery, aiming for clinical trials by the end of 2025. Building on DeepMind’s AlphaFold, it partners with Eli Lilly and Novartis to accelerate R&D. These technologies are not only reducing development time but also increasing success rates by enabling more precise targeting of diseases. Big data analytics further supports trial design, patient recruitment, and real-time monitoring.
As pharmaceutical firms seek greater efficiency and cost-effectiveness, the adoption of AI and data-centric approaches is rapidly becoming a competitive necessity in modern biopharma innovation.
To get more insights about this report Download Free Sample Report
Biopharmaceutical Market Growth Factors
Rising Prevalence of Chronic and Rare Diseases
The rising prevalence of chronic and rare diseases is a significant driver of the global biopharmaceutical industry. As the incidence of conditions such as cancer, cardiovascular diseases, diabetes, and autoimmune disorders continues to grow, the demand for advanced biopharmaceutical treatments increases.
- For instance, the World Health Organization reported that chronic diseases like cardiovascular diseases, cancer, chronic respiratory diseases, and diabetes kill 41 million people annually. Similarly, according to The Lancet, rare diseases affect an estimated 300 million people worldwide, posing diagnostic challenges due to limited awareness and complex genetic factors.
Biopharmaceuticals offer targeted therapies that improve patient outcomes for these complex conditions, driving R&D investments and market growth. This rising disease burden fuels the development and adoption of novel biologics, monoclonal antibodies, and gene therapies globally.
Market Restraint
Complex Regulatory and Approval Pathways across Regions
The biopharmaceutical market faces significant challenges due to complex regulatory and approval pathways that vary widely across different regions. Each country or region has its own stringent regulatory frameworks, which can lead to prolonged approval times and increased costs for biopharmaceutical companies.
Navigating these diverse requirements demands extensive documentation, clinical trial data, and compliance with local standards, creating barriers to swift market entry. Moreover, differences in safety and efficacy evaluation criteria add further complexity, delaying product launches and increasing time-to-market. This regulatory heterogeneity often discourages smaller firms from entering the market and limits patient access to innovative biologic therapies, thereby restraining overall market growth.
Market Opportunities
Development of Novel Biologics
The development of novel biologics for oncology, autoimmune, and neurological diseases presents a significant opportunity in the biopharmaceutical industry. These therapeutic areas have high unmet medical needs, and biologics offer targeted, efficient, and often safer treatment options compared to traditional small-molecule drugs. Advances in genetic engineering, recombinant DNA technology, and antibody-drug conjugates are accelerating innovation in this space.
- For instance, in May 2025, the FDA approved AbbVie’s Emrelis, an antibody-drug conjugate targeting non-squamous non-small cell lung cancer (NSCLC) patients with high c-Met protein levels. In a mid-stage clinical trial, Emrelis demonstrated a 35% overall response rate, offering a more precise alternative to traditional chemotherapy.
As more companies invest in R&D, particularly in immunotherapies and neurodegenerative disease treatments, the demand for novel biologics is expected to surge globally, thereby driving the biopharmaceutical market growth.
Regional Insights
The North American biopharmaceutical market is driven by strong R&D infrastructure and robust healthcare funding. The presence of advanced biotechnology hubs and favorable regulatory frameworks accelerates drug development and approval. Growing investments in personalized medicine and biologics manufacturing further fuel growth. Additionally, increasing collaborations between academia and industry boosts innovation. The region’s well-established healthcare system and high patient awareness contribute to the rapid adoption of novel biopharmaceuticals, supporting sustained market expansion.
U.s. Biopharmaceutical Market Trends
- The US biopharmaceutical industry is the world’s largest, powered by leading biotech firms such as Amgen, Gilead Sciences, and Moderna. The market thrives on advanced gene and cell therapies, exemplified by CAR-T treatments for cancer. Strong FDA regulatory support, vast R&D funding, and an extensive healthcare infrastructure drive innovation. The surge in mRNA vaccine technology, highlighted by COVID-19 vaccines, also fuels growth in this dynamic market.
- Canada’s biopharmaceutical market is rapidly growing, driven by strong government support and increasing R&D investments. For example, companies like Apotex and BioSyent are advancing biosimilar development. The country benefits from a robust healthcare system and initiatives like Canada’s Strategic Innovation Fund, which fosters biotech innovation. Growing demand for personalized medicine and rare disease treatments further boosts market expansion.
Asia-Pacific Biopharmaceutical Market Trends
The Asia Pacific biopharmaceutical industry is expanding rapidly due to improving healthcare infrastructure and rising healthcare expenditure. Increasing patient awareness and growing middle-class populations drive demand for advanced biologics and biosimilars. The region benefits from expanding manufacturing capabilities and favorable government initiatives supporting biotechnology innovation. Emerging markets provide substantial growth opportunities as access to healthcare improves. Strategic partnerships with global biopharma companies accelerate technology transfer and market penetration, making Asia Pacific a vital growth region in the biopharmaceutical sector.
- Japan's biopharmaceutical market is one of the largest in Asia, driven by an aging population and strong government support for innovative therapies. The country leads in regenerative medicine and has fast-tracked approvals for cell and gene therapies. Companies like Takeda and Astellas are investing heavily in biologics and biosimilars, capitalizing on advanced healthcare infrastructure and patient demand for personalized medicine.
- India's market is rapidly expanding due to increasing healthcare access and government initiatives promoting biosimilars and affordable biologics. With a strong generic drug manufacturing base, India is emerging as a global hub for biosimilar production, supported by firms like Biocon and Dr. Reddy’s. The country’s growing middle class and rising chronic disease burden create substantial opportunities for innovative biopharmaceutical therapies.
Europe Biopharmaceutical Market Trends
Europe’s biopharmaceutical industry growth is supported by a well-developed healthcare network and strong regulatory support for innovative therapies. The region benefits from significant public and private investments in R&D and manufacturing capabilities. Increasing focus on biosimilars and orphan drugs is expanding treatment options. Collaborative research initiatives and policy frameworks encouraging innovation further enhance growth. The rising elderly population and growing prevalence of chronic diseases create strong demand for advanced biopharmaceutical products, making Europe a key market.
- The UK’s biopharmaceutical market benefits from a strong life sciences ecosystem and regulatory support post-Brexit. It is a global leader in gene and cell therapies, exemplified by companies like Oxford Biomedica. The NHS’s adoption of innovative biologics and government initiatives such as the Accelerated Access Pathway enhance market growth. The UK’s emphasis on digital health integration also accelerates biopharma innovation and clinical trial efficiency.
- Germany's market for biopharmaceutical is one of Europe’s largest, driven by strong R&D and government support. The country excels in biosimilars and advanced therapies, with companies like BioNTech pioneering mRNA vaccines. Germany’s robust healthcare infrastructure and growing aging population further fuel demand for innovative biologics. Increasing collaborations between academia and industry are also boosting pipeline developments in oncology and autoimmune treatments.
Product Type Insights
Monoclonal antibodies (mAbs) dominate the market due to their high specificity, efficacy, and expanding therapeutic applications. Widely utilized in treating cancer, autoimmune diseases, and infectious disorders, mAbs are driving innovation in targeted therapies. Their ability to bind precisely to disease-associated antigens enhances treatment outcomes with fewer side effects. Ongoing advancements in antibody engineering and biosimilar development further fuel their market share. Leading companies continue investing heavily in mAb research, making this segment a cornerstone of the global biopharmaceutical industry.
Therapeutic Area Insights
The oncology segment leads the market for biopharmaceutical, driven by rising global cancer incidence and a surge in innovative biologic therapies. Biopharmaceuticals, including mAbs, CAR-T cells, and immune checkpoint inhibitors, are transforming cancer treatment paradigms. Increased R&D funding, personalized medicine approaches, and faster regulatory approvals for oncology drugs have accelerated market growth. Companies are prioritizing oncology pipelines due to strong demand and high return potential, making it the most lucrative therapeutic area within the biopharmaceutical landscape.
Manufacturing Type Insights
Contract Development and Manufacturing Organizations (CDMOs) are vital to the market, offering cost-effective and specialized services for biologics production. Biopharma companies increasingly outsource complex manufacturing processes to CDMOs to accelerate time-to-market and manage capacity challenges. CDMOs provide scalability, regulatory expertise, and advanced technologies essential for biologics, biosimilars, and novel therapies. As demand for biologics grows, the reliance on CDMOs is rising, positioning this segment as a strategic enabler of efficiency, flexibility, and innovation in the global biopharmaceutical supply chain.
Technology Insights
DNA recombinant technology is a foundational pillar of the biopharmaceutical industry, enabling the production of complex biologics such as insulin, hormones, vaccines, and monoclonal antibodies. This technology allows scientists to insert human genes into microorganisms to manufacture therapeutic proteins efficiently and safely. Its high precision, scalability, and ability to produce biologically active substances have made it indispensable. Continued advancements are driving novel drug development, improving yields, and reducing production costs, positioning this segment as a key driver of innovation and growth in biopharma.
Distribution Channel Insights
Hospital pharmacies hold a dominant position in the distribution of biopharmaceuticals due to their critical role in managing complex and high-cost biologics. These facilities ensure the safe storage, handling, and administration of injectable therapies, especially for inpatients requiring specialty care. The rising use of biologics in hospital settings for acute and chronic conditions enhances this segment’s relevance. Additionally, collaborations between hospitals and biopharma firms streamline access and improve patient outcomes, further solidifying hospital pharmacies’ leadership in the supply chain.
Company Market Share
Leading companies in the biopharmaceutical market are focusing on expanding their product portfolios through innovative biologics, biosimilars, and gene therapies. They are investing heavily in research and development, forming strategic partnerships, and enhancing manufacturing capabilities to improve efficiency and meet growing demand. Additionally, companies are leveraging advanced technologies like AI and automation to accelerate drug discovery and clinical trials, aiming to strengthen their market position and drive sustainable growth globally.
Pfizer Inc: Pfizer Inc. is a leading global biopharmaceutical company headquartered in New York, USA. Established in 1849, Pfizer has grown into one of the world’s largest pharmaceutical firms, known for its innovation-driven approach and robust product pipeline. In the biopharmaceutical industry, Pfizer plays a pivotal role through its extensive portfolio of biologics, vaccines, and biosimilars. Its strong global presence, focus on innovation, and partnerships with biotech firms make it a dominant force in the evolving biopharmaceutical landscape.
- In May 2025, Pfizer entered into a licensing agreement with Chinese biopharmaceutical company 3SBio to develop and commercialize the experimental cancer drug SSGJ-707 outside of China. The deal includes a $1.25 billion upfront payment, up to $4.8 billion in milestone payments, and a $100 million equity investment in 3SBio. SSGJ-707 is a bispecific antibody targeting PD-1 and VEGF, currently in Phase III trials in China for various cancers, including non-small cell lung cancer and metastatic colorectal cancer.
List of Key and Emerging Players in Biopharmaceutical Market
- Pfizer Inc.
- Roche Holding AG
- Johnson & Johnson
- Amgen Inc.
- Novartis AG
- Merck & Co., Inc.
- AbbVie Inc.
- Bristol-Myers Squibb Company
- Sanofi S.A.
- Gilead Sciences, Inc.

To get more findings about this report Download Market Share
Recent Developments
- May 2025- GSK, a major pharmaceutical company based in the UK, is committing up to $2 billion to acquire efimosfermin, a potential treatment for liver disease, from Boston Pharmaceuticals. The agreement involves an initial payment of $1.2 billion, with the remainder tied to future milestone achievements. Additionally, GSK will pay royalties to Novartis, which originally developed the drug.
- May 2025- Bengaluru-based biopharmaceutical startup Avammune Therapeutics has secured $12 million (around ₹100 crore) in a fresh funding round. The round was co-led by Capital 2B, Shastra VC, and Kotak Lifesciences Fund I, with additional backing from IvyCap Ventures and 1Crowd. The newly acquired capital will be used to accelerate the company’s research and development initiatives, focusing on the advancement of cutting-edge therapies in the biopharma space.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 450.89 Billion |
| Market Size in 2025 | USD 487.99 Billion |
| Market Size in 2033 | USD 918.75 Billion |
| CAGR | 8.23% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Therapeutic Area, By Manufacturing Type, By Technology, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
Explore more data points, trends and opportunities Download Free Sample Report
Biopharmaceutical Market Segments
By Product Type
- Monoclonal Antibodies (mAbs)
- Vaccines
- Recombinant Proteins
- Gene Therapies
- Cell Therapies
- Biosimilars
- Hormones
- Others
By Therapeutic Area
- Oncology
- Infectious Diseases
- Cardiovascular Diseases
- Autoimmune Diseases
- Neurological Disorders
- Metabolic Disorders
- Hematological Diseases
- Rare/Orphan Diseases
- Others
By Manufacturing Type
- In-house Manufacturing
- Contract Manufacturing (CDMOs)
By Technology
- DNA Recombinant Technology
- Monoclonal Antibody Technology
- Cell Culture Technology
- Chromatography
- Fermentation Technology
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Specialty Clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.
Our Clients:










































