Cardiology PoC Diagnostics Market Size, Share & Trends Analysis Report By Product (Cholesterol/Lipid Profile Test, Cardiac Bio/Markers, Coagulation Test, Companion Diagnostics), By Technology (Lateral Flow Test (LFT), Optical Liquid Analysis, Electrical Biochemical Sensors, Molecular Diagnostics), By End User (Hospitals, Diagnostic Laboratories, Home-based Testing, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Cardiology PoC Diagnostics Market Overview
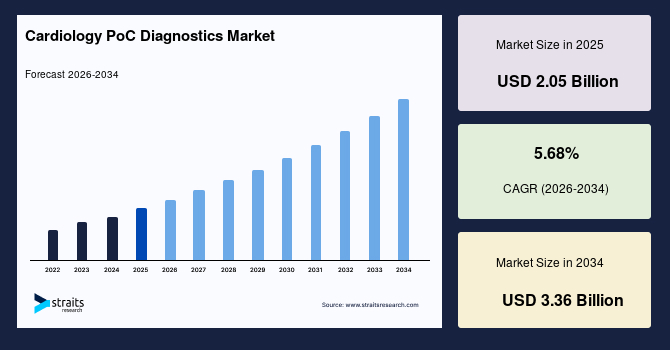
The global cardiology PoC diagnostics market size is estimated at USD 2.05 billion in 2025 and is projected to reach USD 3.36 billion by 2034, growing at a CAGR of 5.68% during the forecast period. Remarkable growth of the market is propelled by the increasing prevalence of cardiovascular diseases, growing demand for rapid and accurate diagnostic solutions, and the shift toward decentralized healthcare models.
Key Market Trends & Insights
- North America holds a dominant share of the global market, accounting for 35.47% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 7.68%.
- Based on Product, the cholesterol/lipid profile test dominated the market in 2025 with a revenue share of 39.42%.
- Based on Technology, the lateral flow test (LFT) dominated the market with a revenue share of 47.25%.
- Based on End User, diagnostic laboratories dominated the market in 2025, with a revenue share of 42.34%.
- The U.S. dominates the global cardiology PoC diagnostics market, valued at USD 624.92 million in 2024 and reaching USD 657.73 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
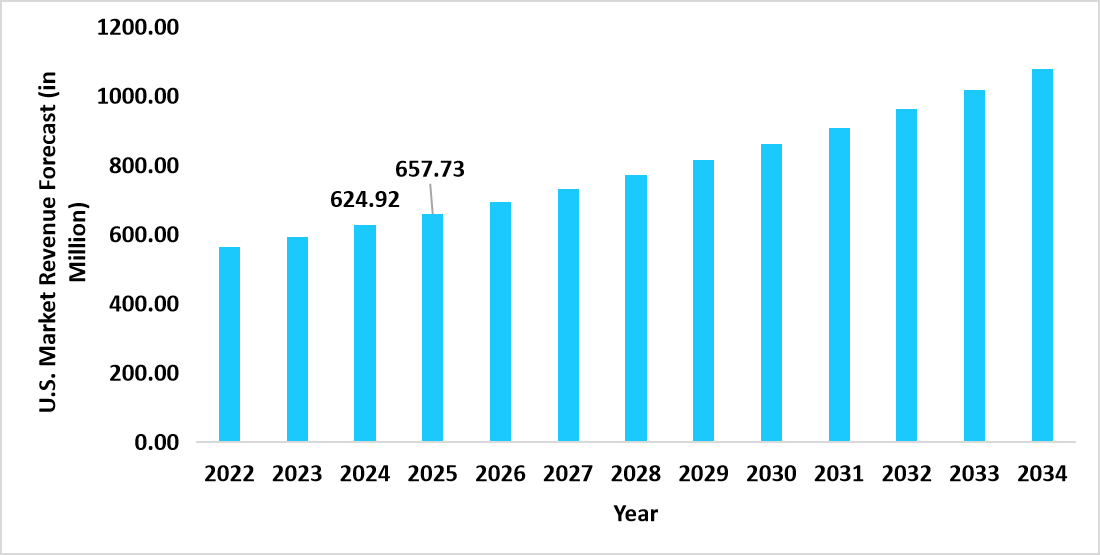
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.05 billion
- 2034 Projected Market Size: USD 3.36 billion
- CAGR (2025 to 2034): 5.68%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The cardiology point-of-care (PoC) diagnostics market refers to the segment of healthcare diagnostics focused on rapid, near-patient testing for cardiovascular conditions to enable timely clinical decisions. It encompasses products such as cholesterol/lipid profile tests, cardiac biomarkers, coagulation tests, and companion diagnostics designed to detect, monitor, and manage cardiac disorders. The market is characterized by the adoption of diverse technologies, including lateral flow tests (LFT), optical liquid analysis, electrical biochemical sensors, and molecular diagnostics, which enhance accuracy and turnaround time of cardiac testing. These diagnostic solutions are utilized across various end users such as hospitals, diagnostic laboratories, home-based testing setups, and other healthcare facilities, aiming to improve cardiac care delivery through faster and more accessible diagnostic results.
Latest Market Trends
Integration of AI and Data Analytics in PoC Cardiac Testing
The adoption of artificial intelligence and data analytics in cardiology point-of-care diagnostics is transforming cardiac assessment accuracy. Machine learning models are being integrated into PoC analyzers to interpret biomarker data such as troponin and BNP with improved precision. These tools enable clinicians to identify abnormal cardiac patterns earlier, streamline decision-making in emergency care, and reduce diagnostic turnaround time. This trend supports the transition toward predictive diagnostics and personalized cardiac treatment planning.
Shift Toward Portable and Patient-Centric PoC Devices
Manufacturers are increasingly focusing on compact and user-friendly PoC cardiac analyzers that can be easily deployed across hospitals, ambulatory centers, and home-care environments. Devices are being designed for portability and simplified operation, enabling faster testing outside traditional laboratory settings. This shift aligns with the broader movement toward decentralized cardiac diagnostics, where patients gain quicker access to biomarker testing and continuous monitoring across multiple points of care.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.05 Billion |
| Estimated 2026 Value | USD 2.16 Billion |
| Projected 2034 Value | USD 3.36 Billion |
| CAGR (2026-2034) | 5.68% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Abbott, Hoffmann-La Roche Ltd, Siemens Healthineers AG, Danaher Corporation, BD |
to learn more about this report Download Free Sample Report
Cardiology PoC Diagnostics Market Driver
Growing Adoption of PoC Cardiac Testing in Preventive Care Frameworks
Healthcare systems are incorporating point-of-care cardiac diagnostics into preventive and early intervention programs. Physicians are leveraging PoC devices for routine cholesterol, troponin, and lipid testing to monitor cardiovascular risk and initiate timely treatment. The use of portable analyzers in corporate wellness and primary care settings has expanded patient participation in preventive cardiac screening. This proactive approach is reducing hospital admissions related to unmanaged cardiac conditions and supporting long-term disease management goals.
Market Restraint
Inadequate Digital Infrastructure in Emerging Regions
Limited digital connectivity and interoperability in developing economies restrict large-scale deployment of PoC cardiac testing systems. Insufficient data exchange between diagnostic devices and healthcare databases hampers real-time reporting and follow-up care. Rural and resource-constrained areas face challenges in maintaining device calibration and data transmission, which affects continuity of cardiac care delivery. These limitations slow the expansion of PoC cardiac diagnostics beyond urban healthcare centers.
Market Opportunity
Expansion of PoC Cardiac Diagnostics Through Telehealth Platforms
The rise of telehealth services offers new pathways for integrating PoC cardiac diagnostics into virtual care ecosystems. Hospitals and outpatient centers are deploying connected PoC analyzers that transmit real-time biomarker results to physicians through telemedicine portals. This advancement enables remote cardiac evaluation and faster clinical intervention for patients outside hospital settings. Growing reimbursement support for tele-cardiology programs is encouraging diagnostic firms to collaborate with telehealth providers, broadening access to on-demand cardiac testing and monitoring worldwide.
Regional Analysis
North America dominated the cardiology point-of-care diagnostics market with the largest revenue share of around 35.47% in 2025. The region’s growth was driven by the integration of rapid cardiac biomarker testing within emergency departments, outpatient clinics, and diagnostic laboratories. Supportive reimbursement frameworks for PoC cardiac assays encouraged hospitals to adopt portable testing platforms, improving clinical workflows for cardiac patients. Collaborations between device manufacturers and hospital networks enabled wider deployment of PoC troponin and BNP analyzers across acute care settings.
The U.S. market expanded with the increased adoption of high-sensitivity troponin PoC assays for early myocardial infarction detection. Hospitals accelerated their transition from centralized laboratory testing to bedside PoC platforms to reduce turnaround time in emergency care. The growing use of portable analyzers in cardiac rehabilitation centers enhanced continuity of care, while partnerships between hospitals and diagnostic companies promoted standardized protocols for cardiac biomarker testing.
Asia Pacific Market Insights
Asia Pacific is anticipated to register the fastest CAGR of 7.68% during the forecast period, fueled by increasing cardiovascular disease prevalence, urbanization, and healthcare infrastructure expansion. Rising government focus on decentralized diagnostics encouraged adoption of PoC cardiac testing in primary and secondary care centers. Investments in local manufacturing and distribution enhanced access to affordable cardiac biomarker analyzers across developing economies.
China’s market advanced with strong policy initiatives promoting early cardiac disease detection through PoC diagnostic systems. Hospitals incorporated portable analyzers for cardiac troponin and lipid testing in outpatient settings to shorten diagnostic delays. Domestic firms developed compact analyzers suited for community health centers, supporting broader implementation of cardiac screening and preventive programs under national healthcare reforms.
Pie Chart: Regional Market Share, 2025
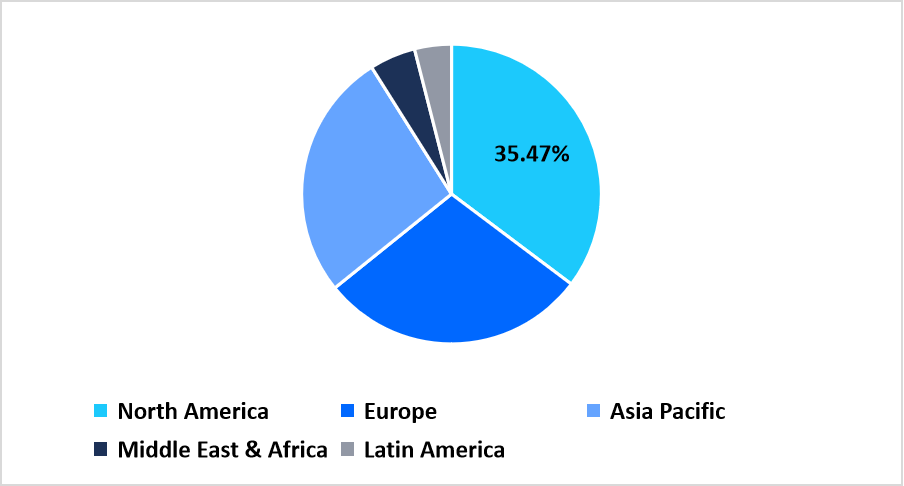
Source: Straits Research
Europe Market Insights
Europe holds a notable share of the cardiology PoC diagnostics market in 2025, driven by established cardiac care networks emphasizing rapid diagnostic triage. The region’s healthcare systems supported PoC testing integration into emergency and ambulatory services to accelerate treatment initiation for acute coronary syndromes. Widespread availability of PoC analyzers across national health systems strengthened clinical readiness for cardiac emergencies.
Germany’s market grows through the expansion of hospital-based PoC programs that prioritize bedside cardiac biomarker testing. Health institutions introduced troponin and D-dimer PoC analyzers within emergency departments to improve patient throughput and diagnostic accuracy. Continuous collaboration between medical device manufacturers and public health authorities supported deployment of standardized PoC platforms aligned with national cardiac care guidelines.
Middle East & Africa Market Insights
The Middle East and Africa demonstrated steady market expansion as countries strengthened cardiac care infrastructure. Governments initiated national screening campaigns and supported PoC diagnostic integration within tertiary and specialty hospitals. The growing number of diagnostic laboratories adopting cardiac biomarker testing platforms contributed to increased utilization of PoC systems across the region.
Saudi Arabia’s market progressed through large-scale healthcare transformation efforts aligned with Vision 2030. Hospitals incorporated PoC cardiac assays for monitoring acute coronary syndromes and post-rehabilitation follow-ups. Strategic alliances between local healthcare providers and global diagnostics companies improved access to PoC testing technology, supporting faster cardiac risk evaluation and patient management.
Latin America Market Insights
Latin America experienced market growth in 2025, supported by national cardiovascular health initiatives and modernization of diagnostic infrastructure. Expansion of public screening programs for heart disease and partnerships with diagnostic manufacturers improved the adoption of PoC analyzers across hospitals and clinics. The shift toward decentralized diagnostics increased accessibility in both urban and rural healthcare facilities.
Brazil’s market expanded as public health agencies introduced cardiac PoC testing programs within primary healthcare centers. Integration of PoC troponin and lipid testing devices into government-led screening projects under the Unified Health System (SUS) improved early diagnosis of cardiovascular conditions. Domestic collaborations with diagnostic companies enhanced device affordability, ensuring broader reach among lower-income populations.
Product Insights
The cholesterol/lipid profile test segment dominated the market in 2025, accounting for 39.42% of total revenue. This dominance was driven by the widespread use of lipid screening in cardiovascular risk assessment and routine health check-ups. The availability of compact PoC lipid analyzers in hospitals and clinics enhanced testing convenience, reducing dependence on centralized laboratories. Growing awareness of early cholesterol management among adults further supported high utilization across diagnostic facilities and primary care settings.
The cardiac biomarkers segment is projected to register the fastest growth, at a CAGR of 6.12% during the forecast period. Increasing adoption of high-sensitivity cardiac troponin and BNP tests for early myocardial infarction detection is fueling expansion. Rising preference for rapid PoC assays in emergency and acute care settings is accelerating this trend. Continuous advancements in immunoassay technology and miniaturised testing devices are making cardiac biomarker testing more accessible across hospital networks and ambulatory care units.
Technology Insights
The lateral flow test (LFT) segment led the market in 2025, representing 47.25% of total revenue. This growth was supported by the widespread availability of LFT-based PoC assays for rapid cardiac biomarker testing in both hospitals and field settings. The segment’s popularity stems from its simple operation, affordability, and short turnaround time, which make it suitable for primary healthcare and emergency use. Adoption of LFTs in developing economies has expanded rapidly due to the convenience of deploying test kits without complex laboratory infrastructure.
The molecular diagnostics segment is expected to grow at the fastest rate, recording a CAGR of 6.35% during the forecast period. The increasing use of molecular testing technologies for identifying genetic and biochemical cardiac risk factors is driving this trend. Demand for high-precision and portable PCR-based PoC devices is rising among hospitals and specialty clinics focusing on advanced cardiovascular diagnostics. Continuous development of multiplex molecular platforms that deliver rapid results supports their integration into both clinical and decentralized care environments.
End User Insights
The diagnostic laboratories segment dominated the market in 2025 with a 42.34% share. High testing throughput, established infrastructure, and wide availability of qualified personnel supported the segment’s leadership. Diagnostic labs continue to serve as the primary centers for comprehensive cardiac biomarker testing and result validation. The integration of PoC analyzers within laboratory workflows improved testing efficiency, reducing result turnaround time for cardiac assessments across healthcare facilities.
The home-based testing segment is projected to record the fastest growth, at a CAGR of 6.78% during the forecast period. The shift toward decentralized diagnostics and the availability of portable PoC analyzers for cholesterol and cardiac marker testing have supported adoption in residential settings. Rising public awareness of preventive cardiac care and the growing popularity of self-testing kits have expanded the patient base for home-use PoC devices. The increasing connectivity of PoC analyzers with mobile health platforms is further supporting early detection and remote cardiac monitoring.
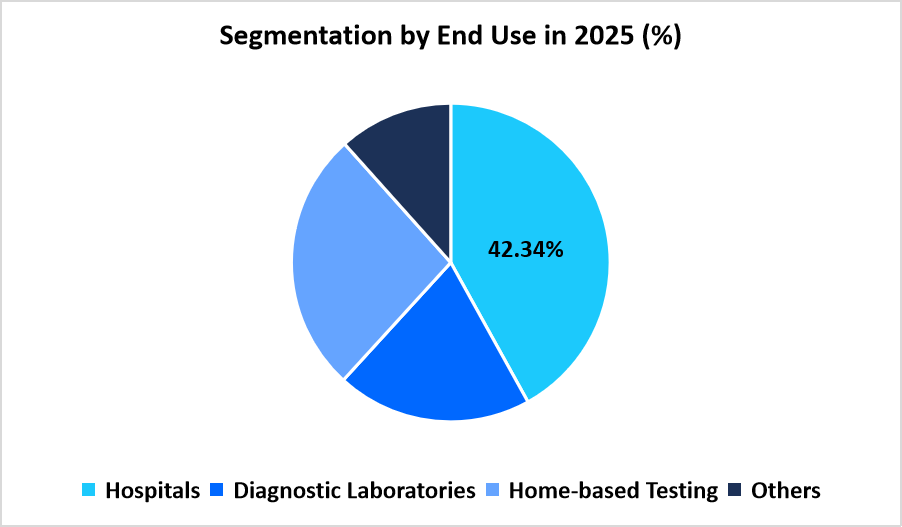
Source: Straits Research
Competitive Landscape
The global cardiology point-of-care (PoC) diagnostics market is moderately fragmented, comprising leading diagnostics giants, specialized cardiac assay developers, and emerging innovators focused on rapid testing, molecular diagnostics, and AI-integrated solutions.
Abbott: An emerging market player
Abbott maintains a strong position in the cardiology PoC diagnostics market with its broad product portfolio, including i-STAT and Alinity platforms, offering rapid cardiac biomarker testing such as troponin, BNP, and D-dimer. The company continues to expand its high-sensitivity cardiac troponin (hs-cTn) assays to emergency and critical care settings.
- In March 2024, Abbott launched an upgraded i-STAT TnI-Next cartridge designed for faster turnaround and enhanced analytical accuracy, strengthening its foothold in hospital and emergency PoC testing.
List of Key and Emerging Players in Cardiology PoC Diagnostics Market
- Abbott
- Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- Danaher Corporation
- BD
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- QuidelOrtho Corporation
- Nova Biomedical, Inc.
- Alfa Scientific Designs, Inc.
- Ortho-Clinical Diagnostics
- Trinity Biotech plc
- Sekisui Diagnostics, LLC
- Nipro Corporation
- Hemocue AB
- DiaSorin S.p.A.
- Randox Laboratories Ltd.
- Meso Scale Diagnostics, LLC
- LifeSign LLC
- Nano-Ditech Corporation
- Others
Strategic Initiatives
- August 2025: Analytics for Life and CorVista Health announced positive results from a clinical study evaluating a non-invasive point-of-care (POC) diagnostic test for detecting elevated pulmonary capillary wedge pressure (PCWP), a key marker in heart failure management. The novel device, used machine learning to analyze patient signals in under four minutes, achieved its primary sensitivity endpoint for identifying PCWP levels associated with both HFpEF and HFrEF. This advancement represented a major step toward replacing invasive catheterisation procedures, strengthening the role of cardiac biomarkers and hospital-based POC diagnostics in advanced heart failure assessment.
- September 2024: Researchers from the European Society of Cardiology (ESC) highlighted a novel POC test (requiring only ~8 minutes on whole blood) for the diagnosis or rule-out of heart attack in the emergency department.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.05 Billion |
| Market Size in 2026 | USD 2.16 Billion |
| Market Size in 2034 | USD 3.36 Billion |
| CAGR | 5.68% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Technology, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Cardiology PoC Diagnostics Market Segments
By Product
- Cholesterol/Lipid Profile Test
- Cardiac Bio/Markers
- Coagulation Test
- Companion Diagnostics
By Technology
- Lateral Flow Test (LFT)
- Optical Liquid Analysis
- Electrical Biochemical Sensors
- Molecular Diagnostics
By End User
- Hospitals
- Diagnostic Laboratories
- Home-based Testing
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































