Cell and Gene Therapy Market Overview
The global cell and gene therapy market size was estimated at USD 37.28 billion in 2025 and is anticipated to grow from USD 45.96 billion in 2026 till USD 250.64 billion in 2034, growing at a CAGR of 23.62% from 2026-2034. The growth is due to the growing prevalence of genetic disorders, which has heightened the demand for effective treatments, further driving the market growth.
Key Market Trends & Insights
- North America held a dominant share of the global cell and gene therapy industry, accounting for 52.16%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 25.86%.
- Based on the Therapy Type, gene therapy is expected to register the fastest CAGR of 25.03%.
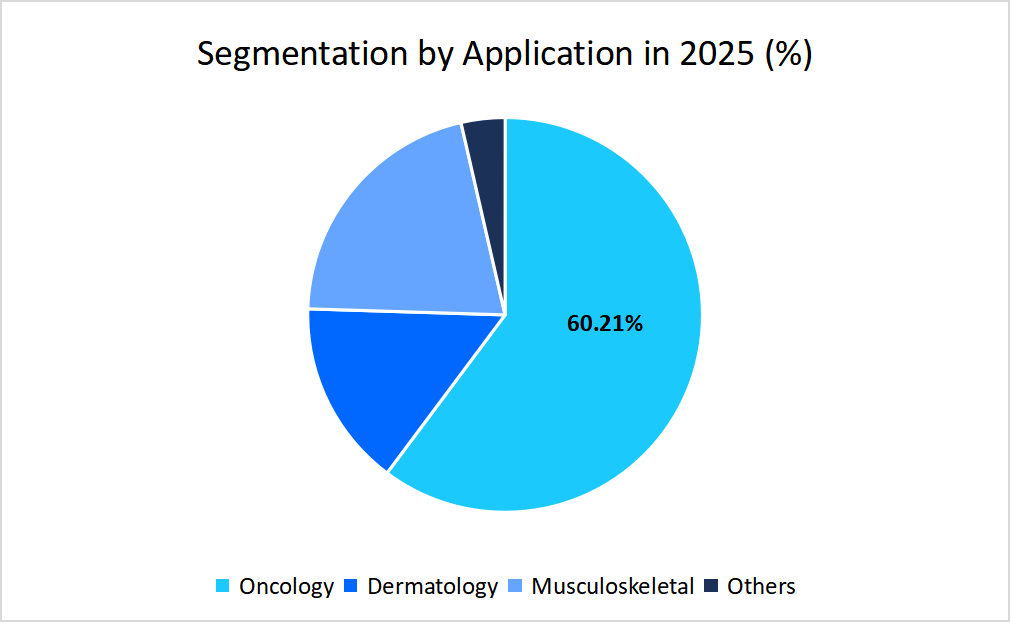
- Based on the Application, the oncology segment held the highest market share of 60.21 % in 2025.
- Based on Manufacturers, the biopharmaceutical and biotechnology companies segment dominated the market in 2025.
- The U.S. dominates the global cell and gene therapy market, valued at USD 14.66 billion in 2024 and reaching USD 18.09 billion in 2025.
Market Size & Forecast
- 2025 Market Size: USD 37.28 billion
- 2034 Projected Market Size: USD 250.64 billion
- CAGR (2025 to 2034): 23.62%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
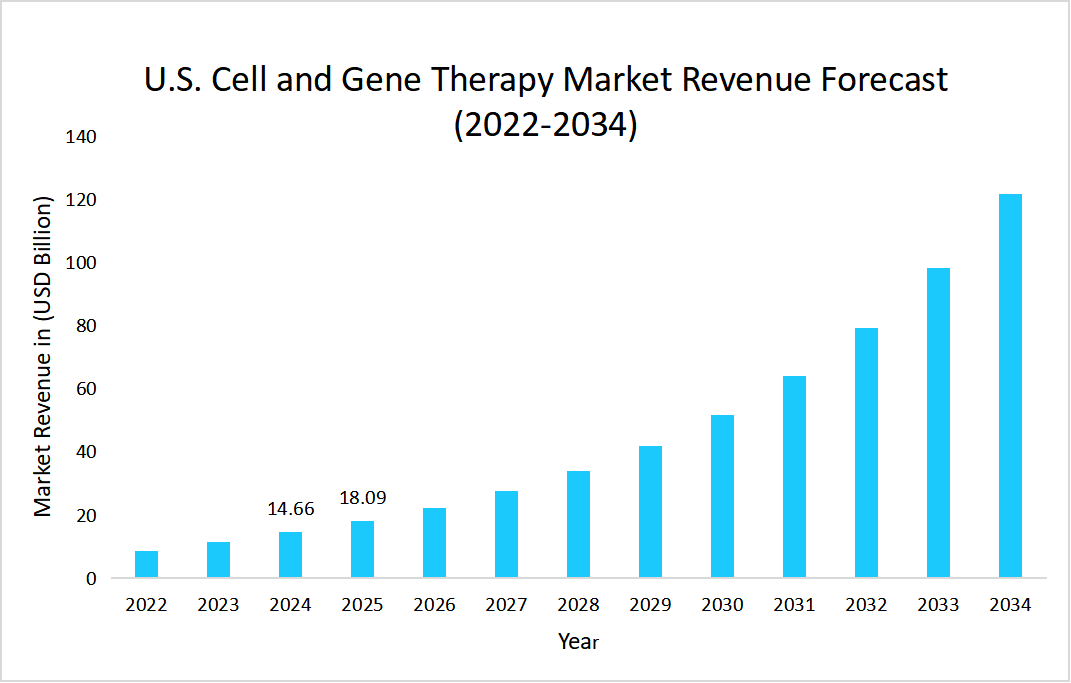
Source: Straits Research Analysis
The market is experiencing well-determined growth due to various factors, such as investments in various therapies and ongoing clinical trials aimed at developing innovative treatments. The following graphs depict the overall investment in regenerative medicines, which includes cell therapy as well as gene therapy, over the period of Q1 2025.
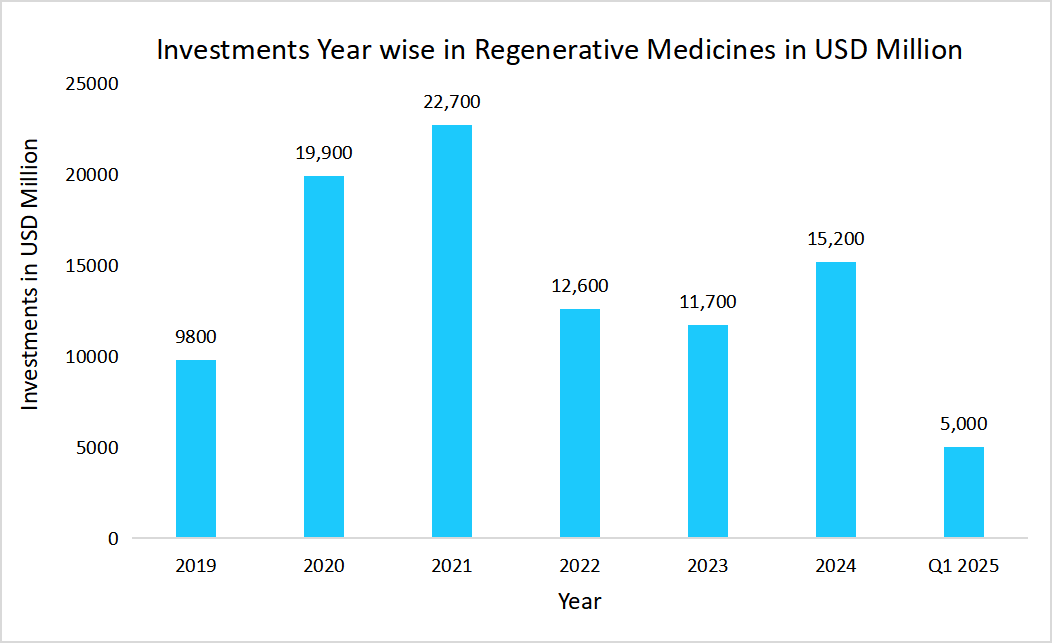
Source: Alliance for Regenerative Medicine
In addition to this, clinical trials also play a significant role in the growth of the market due to their important role in validating the safety, efficacy, and therapeutic potential of new treatments. The table below depicts the data of clinical trials of cell therapy from 2024 to H1 2025.
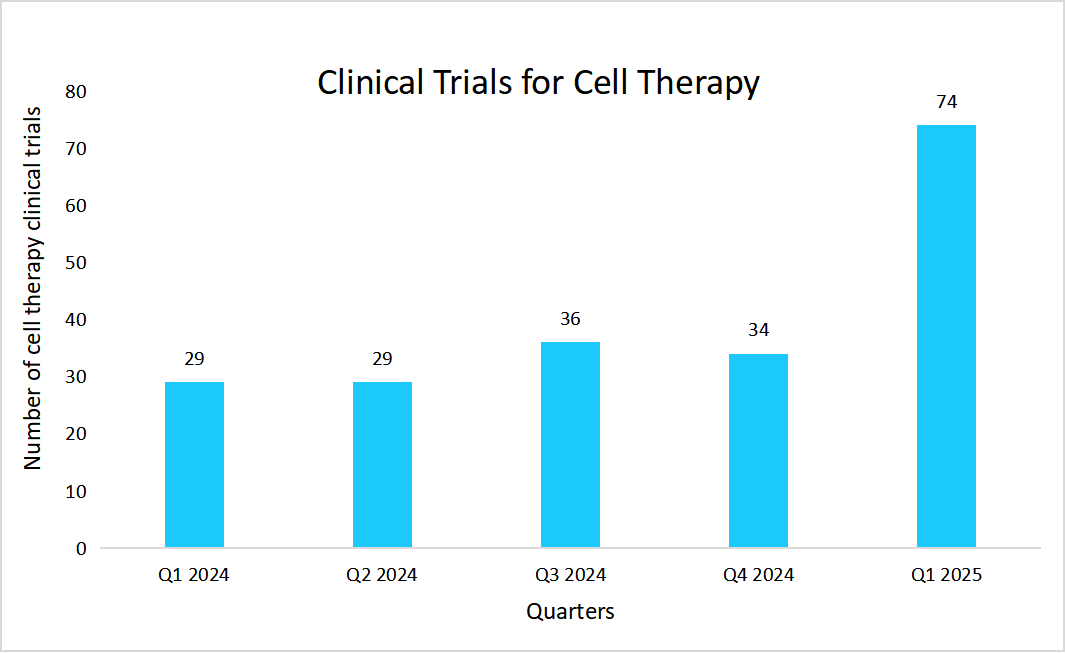
Source: Alliance for Regenerative Medicine
Furthermore, the incorporation of real-world data into the evaluation of cell and gene therapy allows for a more comprehensive understanding of the effectiveness and safety of cell and gene therapy. This evidence-based approach not only enhances patient confidence and clinical decision-making but also supports the sustainable growth and broader adoption of the cell and gene therapy market, ultimately advancing the development of innovative treatments and improving patient access to life-changing therapies.
Market Trends
Shift from Oncology Centric Genetically Modified Cell Therapies to Non-Genetically Modified Therapies for Broader Indications
The clinical focus is shifting from genetically modified cell therapies mainly for oncology towards non-genetically modified cell therapies, which are increasingly being explored for non-oncology indications.
- For instance, in Q1 2025, according to the data reported in the American Society of Gene + Cell Therapy, the following data depicted the increase in the number of non-genetically modified cell therapy trials for non-oncology indications.
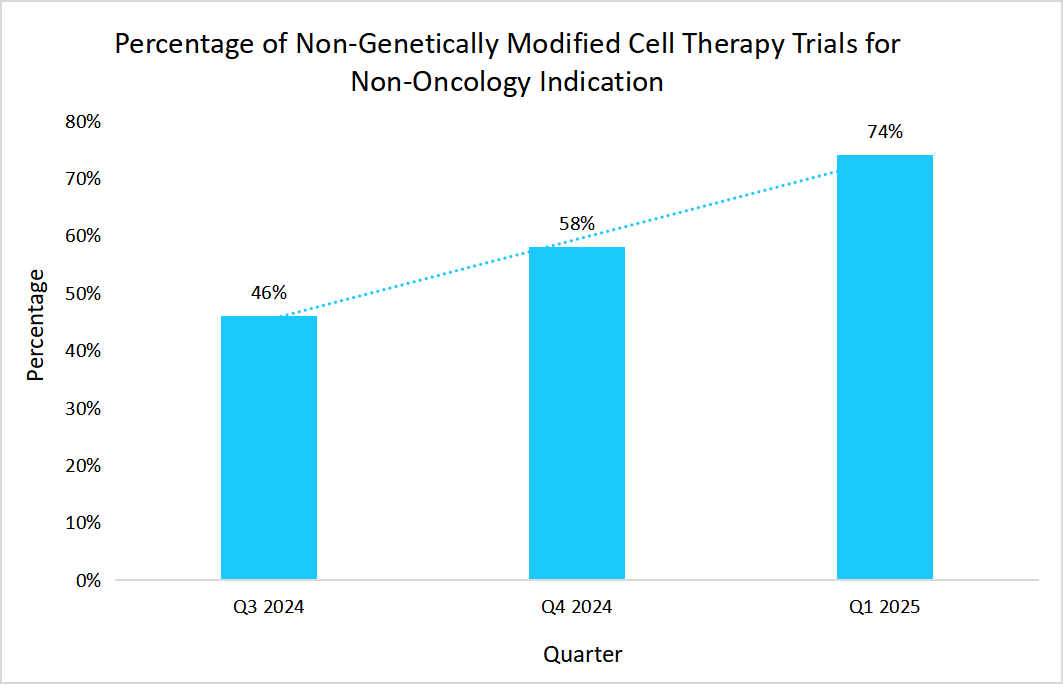
Source: American Society of Gene + Cell Therapy and Straits Analysis
Such a factor provided manufacturers the ability to reach out to the larger patient populations and address the high unmet medical needs in uncommon and chronic diseases.
Expanding Revenue Momentum in Cell and Gene Therapy
The cell and gene therapy market is witnessing a transformative trend, from reliance on a few established blockbusters such as Yescarta and Zolgensma to a new wave of high-growth therapies such as Elevidys, Carvykti, and Breyanzíwith, which recorded triple-digit year-over-year growth in Q1 2025.
Table: Revenue Performance of Leading Cell and Gene Therapies
|
Therapy |
Q1 2025 Revenue (USD Million) |
Increase from Q1 2024 |
|
Yescarta |
386 |
2% |
|
Elevidys |
375 |
179% |
|
Carvykti |
369 |
135% |
|
Zolgensma |
327 |
10% |
|
Breyanzi |
263 |
148% |
|
Vyjuveky |
88 |
95% |
Source: Alliance for Regenerative Medicine
Such momentum illustrated the increasing commercial viability of next-generation therapies and signalled a robust trajectory for continued market expansion in the coming years. It reflected how the success of emerging treatments not only validated their therapeutic potential but also reinforced investor confidence, encouraged broader clinical adoption, and laid the groundwork for sustained growth across the cell and gene therapy landscape.

To get more insights about this report Download Free Sample Report
Market Driver
Progressive Reimbursement Framework for ATMP Therapies
The consistent year-on-year reimbursement of advanced therapy medicinal products (ATMPs) is fueling market growth by enhancing patient access, driving demand, and strengthening industry confidence.
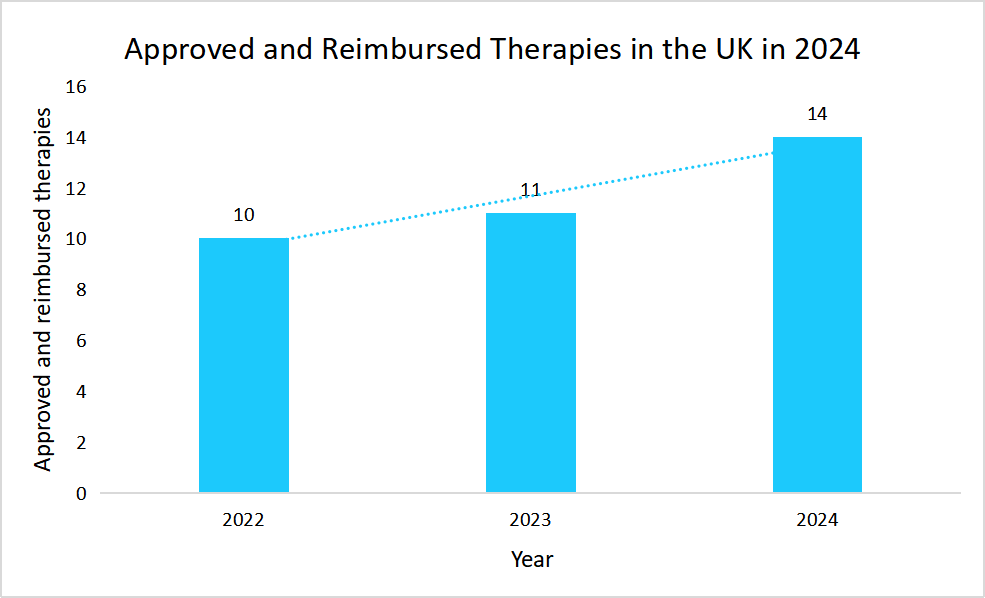
Source: Catapult
Such sustained reimbursement policies in the UK not only ensured wider patient access to advanced therapies but also encouraged continued investment as well as innovation within the cell and gene therapy industry.
Market Restraint
Decline in the Global Private Funding for Cell and Gene Therapy
Decline in the global private funding for cell and gene therapy hinders the market growth as this reduces capital inflows, and also hinders the ability of companies to advance their early-stage research.
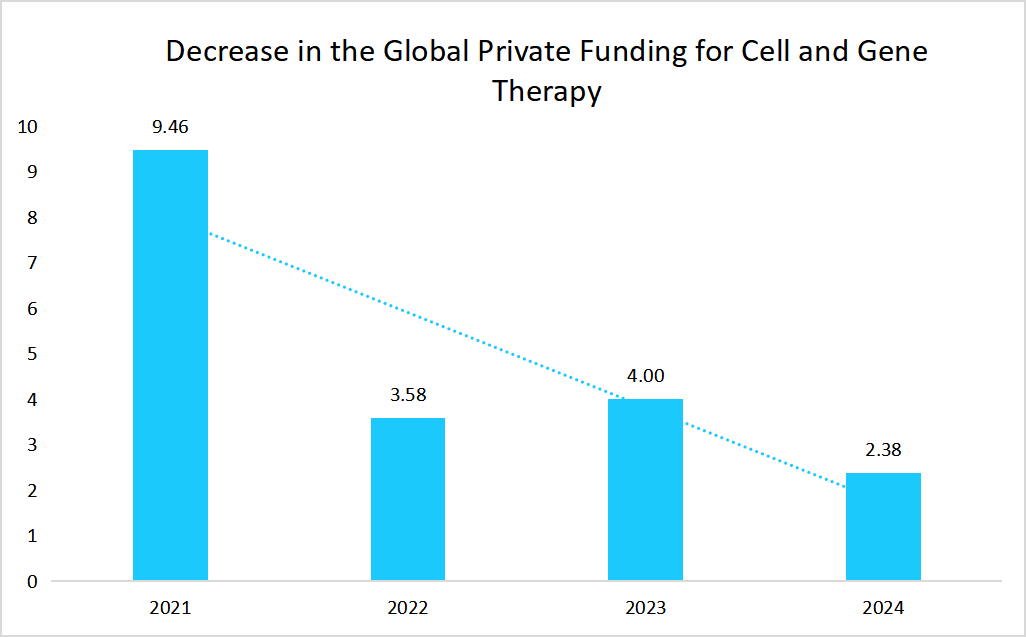
Source: Van Lanschot Kempen analysis
Such a factor directly impacted the manufacturers’ capacity to invest in process optimization, infrastructure expansion, and commercialization strategies, which ultimately slowed innovations and also delayed the entry of new therapies into the market.
Market Opportunity
Growing Dominance of CAR-T Cell Therapies
The growing dominance of CAR-T cell therapies presents a significant market opportunity for manufacturers.
- For example, in 2025, as per the American Society of Gene + Cell Therapy, the CAR-T cell therapy accounted for 50% of all therapies, which depicted a strong demand for specialized manufacturing capabilities.
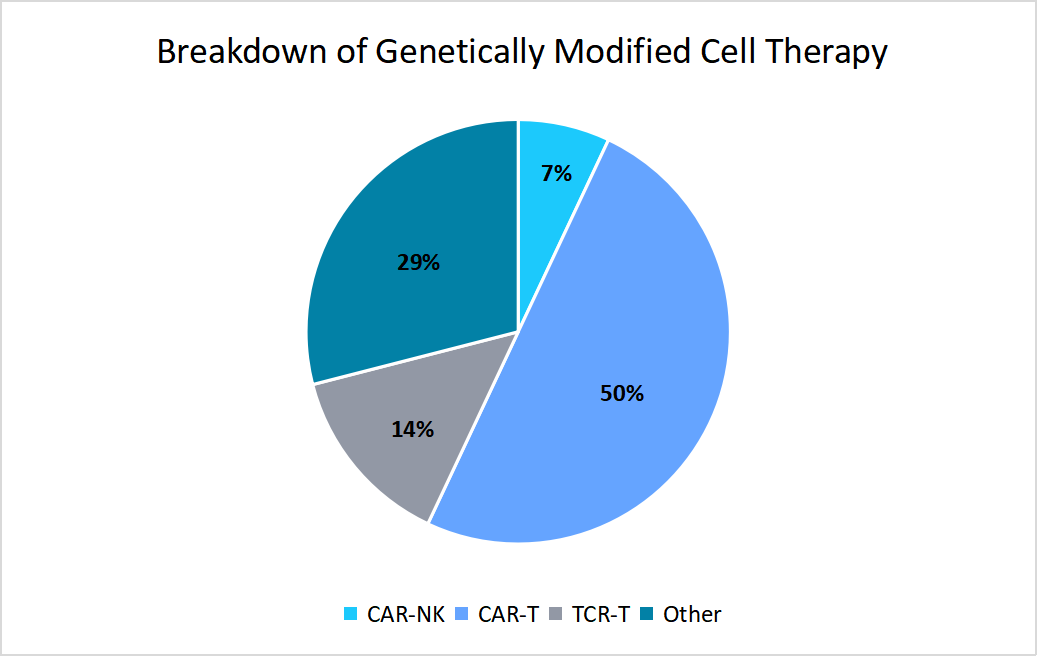
Source: American Society of Gene + Cell Therapy
Such a factor allowed manufacturers to focus more on scaling CAR-T production efficiently, which thereby encouraged investments in advanced CAR-T manufacturing infrastructure and innovation to meet the growing pipeline demands.
Regional Analysis
The North America region dominated the market with a revenue share of 52.16 % in 2025. The growth is attributed to factors such as advanced manufacturing infrastructure, which ensures high-quality production standards and scalability. In addition, strong intellectual property laws that protect innovations in cell and gene therapies also encourage development in the field.
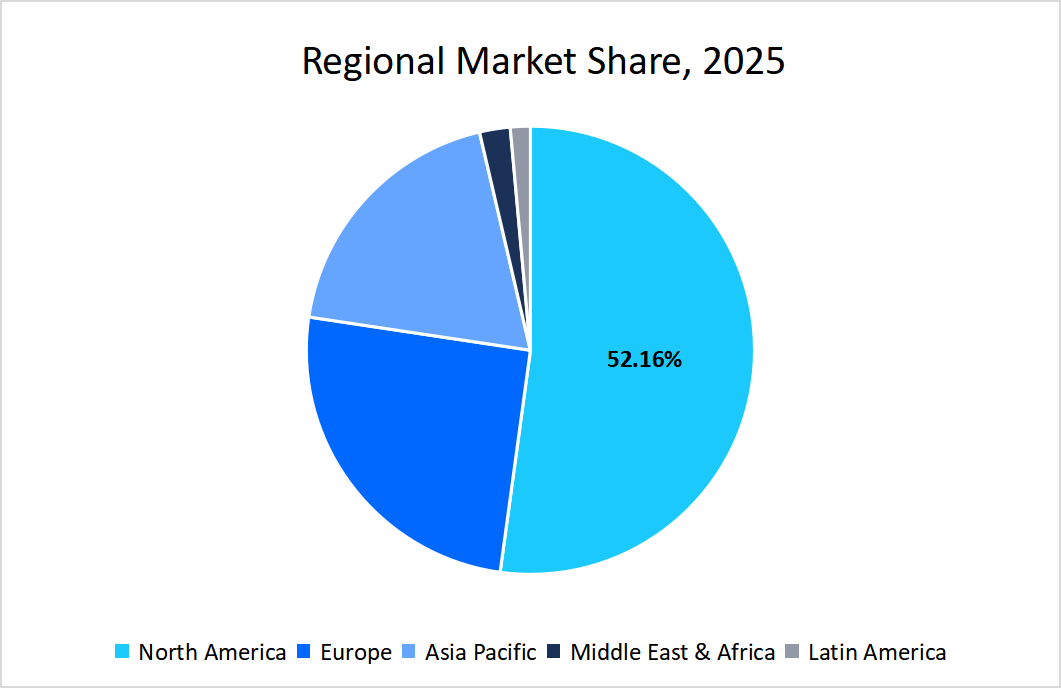
Source: Straits Research Analysis
The U.S. is driving regional growth through supported payer progress. In July 2025, as per the U.S. Centers for Medicare & Medicaid Services, 84% of Medicaid beneficiaries with sickle cell disease participated in CMS’s Cell and Gene Therapy (CGT) Access Model. This initiative facilitated early access to innovative gene therapies, streamlined reimbursement processes, and encouraged broader adoption of advanced treatments.
Asia Pacific Trends Market
The Asia Pacific region is the fastest-growing region with a CAGR of 25.86 % during the forecast timeframe. Countries, such as Japan, South Korea, and Australia, are investing heavily in genomics infrastructure and clinical translation programs to enhance accessibility and accelerate adoption. Moreover, rising demand for innovative treatments for rare genetic disorders and haematological conditions further drives market growth.
India is emerging as a key growth hub within the Asia Pacific, supported by the rise of affordable homegrown CAR-T treatments. In April 2024, as per the PIB, India launched its first homegrown genetic therapy, NexCAR19, developed via the collaborations between IIT Bombay, Tata Memorial Centre, and ImmunoACT, which hence helped India in marking a significant milestone in domestic innovation.
A unique growth driver for South Korea's cell and gene therapy market is its proactive fast-track regulatory system for advanced biopharmaceuticals, which accelerates clinical trial approvals and product launches. Through frameworks like the Advanced Regenerative Medicine and Advanced Biopharmaceuticals Act, South Korea enables quicker market entry for novel therapies by providing early consultations, conditional approvals, and priority review pathways, significantly reducing time to market for innovative CGT products.
Therapy Type Insights
The gene therapy segment is anticipated to grow at a CAGR of 8.21% during 2026 – 2034, owing to the rapid advancements in gene editing technologies. Technologies such as CRISPR-Cas9 and TALENs enable highly precise and targeted modifications of the genome, allowing researchers to correct or replace defective genes responsible for a variety of genetic disorders.
Source: Straits Research Analysis
Application Insights
The oncology segment dominates the market with a revenue share of 60.21% in 2025, supported by the growing use of tumor-specific biomarkers to guide personalized treatments. These biomarkers help doctors identify exactly which therapy is most likely to work for a patient’s unique cancer type. This targeted approach not only improves treatment success but also reduces side effects, making advanced therapies more appealing and effective in real-world cancer care.
Manufacture Insights
The Biopharmaceutical and biotechnology companies segment dominated the market in 2025. This growth is attributed to the strategic collaborations with academic institutions as well as healthcare facilities, which enable rapid translation of research into commercially viable therapies. Such partnerships facilitate access to specialized expertise and advanced laboratory infrastructure that further accelerate the development, testing, and approval of innovative cell and gene therapies.
Competitive Landscape
The global cell and gene therapy market is highly fragmented in nature due to the presence of numerous biotechnology startups, academic spin-offs, and mid-sized companies alongside a few major pharmaceutical players.
Epicrispr Biotechnologies, Inc.: An emerging market player
Epicrispr Biotechnologies, Inc. develops therapies to dynamically control gene expression as well as to treat complex diseases.
- In April 2025, Epicrispr Biotechnologies, Inc. announced the U.S. FDA had cleared its experimental epigenetic therapy for clinical trials across the U.S. It is the first in-human trial that used epigenetic editing for facioscapulohumeral muscular dystrophy (FSHD) condition, representing a paradigm shift in the therapeutic landscape.
List of Key and Emerging Players in Cell and Gene Therapy Market
- Novartis AG
- Amgen Inc.
- Vericel Corporation
- Gilead Sciences, Inc.
- Bluebird Bio, Inc.
- Bristol-Myers Squibb Company
- Biomarin
- Johnson & Johnson and its affiliates
- uniQure NV.
- Celgene Corporation
- ImmunoACT
- Cellectis
- Astellas Pharma Inc.
- Atara Biotherapeutics, Inc.
- Orchard Therapeutics plc
- Sana Biotechnology
- Bayer AG
- Hoffmann-La Roche Ltd
- Krystal Biotech, Inc
- ALLOGENE THERAPEUTICS

To get more findings about this report Download Market Share
Recent Development
- April 2025: XellSmart Biopharmaceutical Co., Ltd. announced that the U.S. FDA cleared its universal allogeneic off-the-shelf iPS-derived dopaminergic neural progenitor cell therapy for the treatment of Parkinson's disease, which is considered the second most common neurodegenerative disease worldwide.
- April 2025: Bio-Techne announced the FDA approval of Zevaskyn, the first autologous cell-based genetic therapy for patients with the recessive dystrophic epidermolysis bullosa, a rare and severely debilitating skin disorder characterized by fragile skin that blisters easily.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 37.28 billion |
| Market Size in 2026 | USD 45.96 billion |
| Market Size in 2034 | USD 250.64 billion |
| CAGR | 23.62% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Therapy Type, By Application, By Manufacturers |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
Explore more data points, trends and opportunities Download Free Sample Report
Cell and Gene Therapy Market Segments
By Therapy Type
-
Cell Therapy
-
CAR-T
- Autologous CAR-T
- Allogeneic CAR-T
- CAR-NK
- B-Cell
- Other
-
CAR-T
-
Gene Therapy
-
Viral
- AAV
- Lentiviral vectors
- Others
- Non-viral
-
Viral
By Application
- Oncology
- Dermatology
- Musculoskeletal
- Others
By Manufacturers
- Biopharmaceutical and biotechnology companies
- Pharmaceutical companies
- CDMOs/CMOs
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.
Our Clients:










































