Clinical Trials Support Software Solutions Market Size, Share & Trends Analysis Report By Solution (Electronic Clinical Outcome Assessment, Electronic Data Capture & CDMS, Clinical Analytics Platforms, Electronic Investigator Site File, Safety solutions, Clinical Trial Management System, Randomization and Trial Supply Management, Electronic Trial Master File, Clinical data integration platforms, Payments/Investigator Payments Solutions, Other Solutions), By Delivery Mode (Cloud-based, On-premise), By Phase (Phase I, Phase II, Phase III, Phase IV), By End Use (Healthcare Providers, Contract Research Organizations, Academic & Research Institutions, Pharmaceutical & Biopharmaceutical Companies, Medical Device Manufacturers) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Clinical Trials Support Software Solutions Market Size
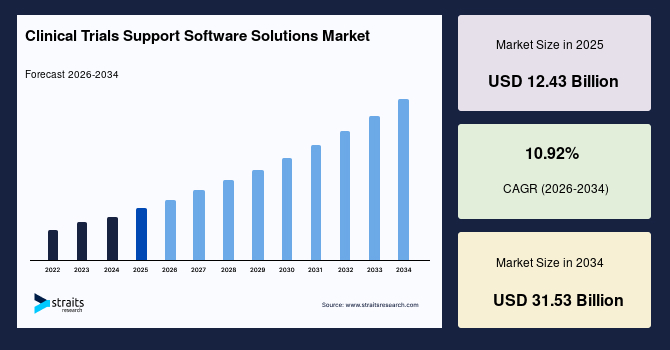
The clinical trials support software solutions market size was valued at USD 12.43 billion in 2025 and is estimated to reach USD 31.53 billion by 2034, growing at a CAGR of 10.92% during the forecast period (2026-2034). Clinical trial support software is used across decentralized trials, eConsent, and trial management. With an increased volume of clinical trials, CROs and biopharmaceutical companies are integrating digital solutions, which is expected to boost the market in the coming years.
Key Market Insights
- North America dominated the clinical trial support software solutions market with the largest market share of 47.30% in 2025.
- The Asia Pacific region is estimated to grow at a CAGR of 12.74% during the forecast timeframe.
- By solution, the clinical trial management system segment dominated the market with a revenue share of 18.79% in 2025.
- By delivery mode, the on-premise segment is estimated to grow at a CAGR of 11.86% during 2026-2034.
- By phase, the phase III segment dominated the market in 2025 with a revenue share of 51.59%.
- Based on end use, the contract research organizations segment dominated the market in 2025.
- The US clinical trial support software solutions market size was valued at USD 5.35 billion in 2025 and is projected to reach USD 5.92 billion in 2026.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 12.43 Billion |
| Estimated 2026 Value | USD 13.76 Billion |
| Projected 2034 Value | USD 31.53 Billion |
| CAGR (2026-2034) | 10.92% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Oracle Health Sciences, Veeva Systems, Thermo Fisher Scientific Inc., IQVIA, IBM Watson Health |
to learn more about this report Download Free Sample Report
Clinical Trials Support Software Solutions Market Trends
Shift toward decentralized and hybrid clinical trials
A major trend in the market is the shift toward decentralized and hybrid trials, driven by sponsors integrating eConsent, eCOA, remote monitoring, and direct-patient logistics within unified platforms. Leading manufacturers such as Medidata and Veeva are expanding native support for home health visits and wearable data integration, aligning with peer-reviewed clinical research demonstrating improved patient retention, diversity, and protocol adherence in decentralized trial models.
Integration of real-world evidence into trial software workflows
The integration of real-world evidence from electronic health records, registries, and claims data into clinical trial support platforms enables sponsors to augment traditional trial data with real-world insights. This enhances protocol design, accelerates regulatory acceptance, and improves external validity of outcomes, with eClinical systems evolving to support structured RWE ingestion, governance, and analytics across study phases.
Clinical Trials Support Software Solutions Market Drivers
Surge in outsourcing to specialized CRO software platforms drives market
The accelerated outsourcing of clinical trial operations to specialized CROs, which depend on advanced software platforms to manage complex, multinational studies efficiently, is driving the market growth. As pharmaceutical companies contract out trial design, recruitment, data management, and regulatory reporting, demand for integrated clinical trial support solutions grows, exemplified by Syneos Health’s collaboration with uMotif to embed eCOA/ePRO capabilities into trial execution workflows.
Expansion of mobile health integration in clinical trials boosts market growth
The integration of wearable technology and mobile health solutions enables the collection of continuous patient data. For example, the mSToPS (mActive Surveillance for Pre-clinical Atrial Fibrillation) trial, published in JAMA Cardiology, utilized the wearable ECG patch data streamed into centralized trial systems to identify arrhythmia. Likewise, the use of Fitbit and Apple Watch data in EDC and analytics systems to remotely track patient outcomes is becoming more prevalent in Roche’s DiGA related trials. Such integrations increase the richness of the data, decrease the burden on sites, and increase the need for software solutions that handle such data streams and drive the market growth.
Market Restraints
Data privacy & data transfer restrictions hamper adoption
A key restraint is tightening data privacy regulations that limit clinical data flows, complicating multinational trial operations. For example, the EU’s updated General Data Protection Regulation enforcement and India’s evolving digital health data rules have forced sponsors to invest heavily in localized data hosting and compliance audits. This increases implementation costs and delays deployment of integrated trial platforms across regions, as highlighted in regulatory science literature on clinical data governance.
Market Opportunities
Expansion of complex adaptive and platform trial designs offers lucrative growth opportunities
The rapid expansion of adaptive and platform trial designs, which require advanced analytics, protocol versioning, and integrated data orchestration, presents a new opportunity for market growth. Manufacturers such as Medidata have expanded Rave and Designer capabilities to support master protocols, basket, and umbrella trials, aligning with methodologies published in The New England Journal of Medicine. These complex trials increase reliance on sophisticated support software across oncology and rare disease pipelines.
Technological Landscape
- Veeva Vault CTMS is a cloud-native platform that supports site management, monitoring budgets, and reporting in real time.
- Rave, a part of Medidata Clinical Cloud, offers real-time insights, risk-based monitoring, and integrated trial operations.
- Oracle Siebel Clinical CTMS offers trial tracking, budgeting, and resource allocation.
Regional Analysis
The clinical trial support software solutions market in North America had a market share of 47.30% in 2025. This growth is driven by the region’s concentration of large precision medicine and rare disease trial hubs that demand highly tailored software integrations for handling complex genomic, biomarker, and longitudinal real-world data.
Canada is the fastest-growing country in North America, stimulated by the emergence of AI-enabled digital twin modeling initiatives led by Canadian research and technology firms. These platforms create virtual patient counterparts that predict individual disease progression and treatment responses, assisting sponsors in refining trial design, improving statistical power, and shortening trial timelines.
Asia Pacific
Asia Pacific is emerging as the fastest-growing region with a CAGR of 12.74% from 2026 to 2034, owing to the region’s early readiness for decentralized clinical trials, driven by strong patient acceptance of digital health tools. High participation in remote monitoring and virtual visits encourages sponsors to adopt advanced clinical trial software across the region.
The clinical trials support software solutions market in Australia is augmented by the increasing adoption of trial software solutions. The Australian Teletrial model integrates remote trial participation technologies to expand access in rural and regional areas. This approach, supported by national funding, requires advanced digital platforms to manage remote monitoring, telehealth visits, and decentralized data capture.
Europe
The Europe clinical trials support software solutions market growth is supported by the willingness to adopt advanced software solutions. For instance, the implementation of the EHR4CR platform allows researchers to query millions of electronic health records across European hospitals for trial feasibility and patient matches while fully complying with strict EU privacy laws. This capability sharply accelerates patient identification and site selection in multi‑country trials.
The market growth in Germany is supported by its advanced health research data infrastructure under the Medical Informatics Initiative, which integrates routine care and research data across university hospitals into interoperable networks. This enables seamless, secure access to rich clinical datasets for trial patient matching and analytics, boosting demand for sophisticated eClinical platforms.
Latin America
The Latin America clinical trials support software solutions market is witnessing steady growth. The PanAmerican Health Organization’s regional clinical trials portal creates a centralized framework for ethical oversight, shared data standards, and coordinated trial documentation across multiple countries. This regional harmonization encourages sponsors to deploy advanced software systems that meet unified regulatory and reporting expectations across the Americas.
The Brazilian clinical trials support software solutions market is experiencing growth driven by a positive push by the government. The Brazilian Health Data Network (RNDS), a government-supported interoperable platform that unifies public and private healthcare data under one digital ecosystem. This integration enables more efficient protocol planning, patient identification, and real-time data exchange across sites.
Middle East and Africa
The Middle East and Africa clinical trials support software solutions market is expanding due to the expansion of specialized oncology and rare disease centers, which require advanced digital platforms to manage multi-site patient data and ensure regulatory compliance across diverse healthcare systems, substantially increasing adoption of sophisticated trial management solutions in the region.
The clinical trials support the software solutions market growth in South Africa, which is stimulated by favorable initiatives. The South African 110,000 Human Genome Pilot Program integrates large‑scale whole genome sequencing into clinical research workflows. This initiative generates extensive longitudinal genetic data that requires advanced digital systems for secure data management, thereby driving market growth.
Solution Insights
The clinical trial management system segment accounted for the largest clinical trial support software solutions share of 18.79% in 2025. This growth is attributed to the emergence of modular plug-and-play microservices that support decentralized and hybrid trial models. This helps sponsors to add telemedicine, wearable data feeds, and remote monitoring modules easily in the model.
The payments/investigator payments solutions segment is projected to register a CAGR of 11.44% during the forecast period, owing to growing demand for currency reconciliation and auditability in global clinical finances that automates cross-border tax compliance and investigator compensation tracking. Thus, strong demand from CROs for investigator payment solutions is expected to propel the clinical trial support software solutions market throughout the forecast period.
Delivery Mode Insights
The cloud-based segment dominated the market in 2025, owing to the increasing regulatory endorsement of continuous, cloud-based audit trails and real-time compliance reporting for sponsors and CROs to generate regulatory documentation across international jurisdictions without manual reconciliation automatically.
The on-premise segment is estimated to grow at a CAGR of 11.86% during the clinical trials support software solutions market forecast. This growth is supported by the preference among biopharmaceutical organizations for hosting proprietary AI-augmented trial optimization engines within internal firewalls, enabling in-house model training on sensitive patient and protocol datasets without exposing intellectual property to external cloud environments.
Phase Insights
The phase III segment dominated the market in 2025 with a revenue share of 51.59%, due to increasing requirements to manage patient reimbursement and site budget forecasting simultaneously with trial execution. Phase III involves hundreds of sites and thousands of participants, making integrated financial operational coordination essential and driving higher adoption of advanced trial support software.
The phase I segment is expected to register the fastest CAGR growth during the clinical trials support software solutions market forecast. This growth is driven by the rapid adoption of adaptive first-in-human study designs, which require dose escalation decisions, intensive safety data monitoring, and protocol adjustments, which, in turn, boost segment growth.
End Use Insights
The contract research organizations segment dominated the market in 2025, driven by their requirements to manage multiple clinical trials for different sponsors at the same time. Advanced software assists CROs in standardizing processes, improving coordination across sites, and delivering faster and more effective trial execution.
The healthcare providers segment is expected to register the fastest growth during the forecast timeframe. Healthcare providers are driving rapid adoption of clinical trial support software by integrating trials within EHR workflows and expanding internal research investments. Moreover, the integration of AI solutions to automate patient screening and cohort identification improves recruitment accuracy.
| SEGMENT | INCLUSION | DOMINANT SEGMENT | SHARE OF DOMINANT SEGMENT, 2025 |
|---|---|---|---|
|
SOLUTION |
|
Clinical Trial Management System |
18.79% |
|
DELIVERY MODE |
|
Cloud-based |
XX% |
|
PHASE |
|
Phase III |
51.59% |
|
END USE |
|
Contract Research Organizations |
XX% |
|
REGION |
|
North America |
47.30% |
Regulatory Bodies Governing Clinical Trials Support Software Solutions Market
| REGULATORY BODY | COUNTRY/REGION |
|---|---|
|
US Food & Drug Administration |
US |
|
European Medicines Agency |
Europe |
|
Pharmaceuticals and Medical Devices Agency |
Japan |
|
Central Drugs Standard Control Organisation |
India |
|
Medicines and Healthcare products Regulatory Agency |
UK |
Competitive Landscape
The clinical trials support software solutions market is moderately competitive, with a mix of established technology providers and specialized clinical software vendors. Key players consider factors such as platform integration, AI-driven analytics, cloud-based deployment, and seamless interoperability to compete in the market and strengthen their position. Emerging players are competing on automation-led differentiation, superior user experience, decentralized & hybrid trial readiness, and pricing flexibility. Emerging trends in the market include widespread integration of AI, SaaS-first solutions, and interoperable platforms.
List of Key and Emerging Players in Clinical Trials Support Software Solutions Market
- Oracle Health Sciences
- Veeva Systems
- Thermo Fisher Scientific Inc.
- IQVIA
- IBM Watson Health
- Parexel International
- Clario
- Dassault Systèmes
- RealTime Software Solutions
- Castor EDC
- OpenClinica
- Medrio
- Viedoc
- Cloudbyz
- Jeeva Trials
- eClinical Solutions
- ArisGlobal
- BioClinica
- MasterControl
- Anju Software
- PHARMASEAL
Latest News on Key and Emerging Players
| TIMELINE | COMPANY | DEVELOPMENT |
|---|---|---|
|
November 2025 |
RealTime |
The company launched EDC Connect, a feature designed to eliminate duplicate data entry between eSource and EDC systems. This addresses one of the biggest inefficiencies in clinical data workflows. |
|
October 2025 |
Thermo Fisher Scientific Inc. |
The company agreed to acquire Clario Holdings, Inc. to strengthen its software-enabled clinical trial services across biotech and pharmaceutical customers. |
|
October 2025 |
eClinical Solutions |
The company received the SCDM Innovation Award for Elluminate Assist, a generative AI-powered assistant within its clinical data cloud. |
|
September 2025 |
IQVIA |
IQVIA launched its Clinical Trial Financial Suite an AI-enabled platform orchestrating all financial aspects of clinical trials. |
|
September 2025 |
Anju Software |
The company launched Clinexa, an AI ecosystem of virtual assistants that transform clinical protocols. |
|
September 2025 |
RealTime eClinical Solutions |
The company was recognized with the Innovation Award (Digital Platforms) and the Marketing/Brand Innovation Award at the Clinical Trials Arena Excellence Awards |
|
July 2025 |
PHARMASEAL and Viedoc |
The companies partnered to co-develop an interoperable system with advanced data capture capabilities. |
Source: Secondary Research
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 12.43 Billion |
| Market Size in 2026 | USD 13.76 Billion |
| Market Size in 2034 | USD 31.53 Billion |
| CAGR | 10.92% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Solution, By Delivery Mode, By Phase, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Clinical Trials Support Software Solutions Market Segments
By Solution
- Electronic Clinical Outcome Assessment
- Electronic Data Capture & CDMS
- Clinical Analytics Platforms
- Electronic Investigator Site File
- Safety solutions
- Clinical Trial Management System
- Randomization and Trial Supply Management
- Electronic Trial Master File
- Clinical data integration platforms
- Payments/Investigator Payments Solutions
- Other Solutions
By Delivery Mode
- Cloud-based
- On-premise
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End Use
- Healthcare Providers
- Contract Research Organizations
- Academic & Research Institutions
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Manufacturers
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































