Competent Cells Market Size, Share & Trends Analysis Report By Type (Chemically Competent Cells, Electrocompetent Cells, Ultracompetent Cells), By Application (Cloning, Protein Expression, Others), By End Use (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Competent Cells Market Overview
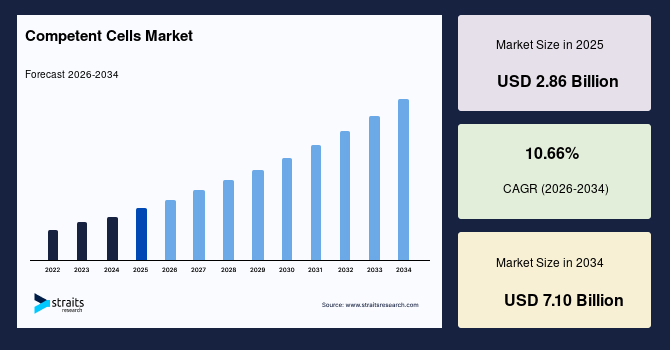
The global competent cells market size is valued at USD 2.86 billion in 2025 and is estimated to reach USD 7.10 billion by 2034, growing at a CAGR of 10.66% during the forecast period. The consistent market growth is supported by the rising scalability of advanced cloning and expression workflows across research and commercial laboratories.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 38.24% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 12.66%.
- Based on type, the chemically competent cells segment held the highest revenue market share of 45.67% in 2025.
- Based on application, the cloning segment dominated the market in 2025 with a revenue share of 45.12%.
- By end use, the pharmaceutical and biotechnology companies segment held the largest market share of 42.32% in 2025.
- S. dominates the competent cells market, valued at USD 898.30 million in 2024 and reaching USD 990.19 million in 2025.
Table: U.S. Competent Cells Market Size (USD Million)
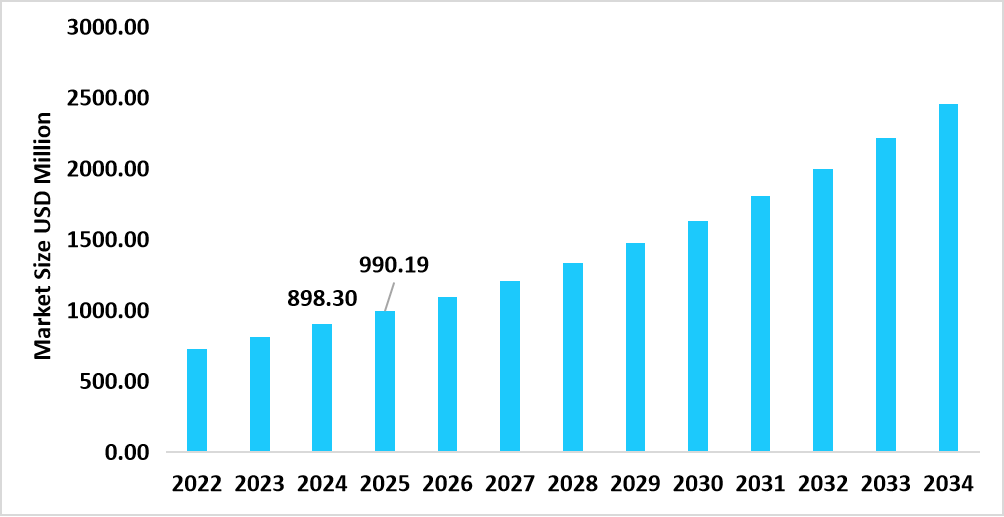
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 2.86 billion
- 2034 Projected Market Size: USD 7.10 billion
- CAGR (2026-2034): 10.66%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The competent cells market encompasses the production and distribution of bacterial cells that are preconditioned to uptake foreign DNA, forming a foundational toolset for molecular biology, genetic engineering, and recombinant production workflows. These cells, available as chemically competent, electrocompetent, and ultracompetent variants, enable researchers to perform a wide spectrum of DNA manipulation tasks, including routine cloning, subcloning, phage display library construction, toxic or unstable DNA handling, and high-throughput construct assembly. Beyond cloning, competent cells support applications such as protein expression, mutagenesis, single-stranded DNA generation, lentiviral vector plasmid preparation, and large plasmid transformation, making them essential across academic research, biopharmaceutical development, and commercial biotechnology operations. The market serves diverse end users ranging from pharmaceutical and biotechnology companies conducting construct design and production scale engineering to academic institutes advancing fundamental research, supported by ancillary segments that utilise competent cells for specialized laboratory procedures and genetic studies.
Latest Market Trends
Shift from standard chemical workflows to precision-tuned transformation formats
A key trend in the competent cells market is the shift from traditional chemical transformation procedures to precision-tuned formats that offer greater control over transformation variables. Laboratories are adopting strain-specific buffers, controlled incubation cycles, and calibrated thermal profiles that reduce procedural variability and increase uniformity across plasmid assembly tasks. This movement toward refinement reflects the growing preference for transformation setups that support consistent outcomes in complex gene-assembly projects, enabling researchers to manage demanding construct designs with greater procedural stability.
Shift from manual colony-screening steps to semi-automated validation pipelines
The major trend is a shift from manual colony selection and screening routines to semi-automated validation pipelines that integrate imaging modules, colony-picking arms, and software-guided verification. Research groups are adopting these systems to streamline the identification of successful transformants, particularly during library construction and multi-variant testing. This transition supports smoother progression from transformation to downstream analysis and reduces the time researchers spend on repetitive screening tasks, enabling laboratories to expand project throughput with clearer workflow continuity.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.86 Billion |
| Estimated 2026 Value | USD 3.16 Billion |
| Projected 2034 Value | USD 7.10 Billion |
| CAGR (2026-2034) | 10.66% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific Inc., New England Biolabs, Agilent Technologies, Inc., Merck KGaA, Takara Bio Inc. |
to learn more about this report Download Free Sample Report
Competent Cells Market Driver
Rising preference for host strains tailored for genome-editing constructs
A key driver for the competent cells market is the rising preference for host strains customised for genome-editing construct assembly. Research teams are increasingly working with multi-component CRISPR systems, base-editing plasmids, and modular repair templates that require stable propagation within specialised bacterial hosts. Demand for strains engineered for complex DNA stability, recombination control, and high-capacity plasmid maintenance is accelerating procurement activity across research institutions. As laboratories expand genomic engineering projects, the consumption of competent cells aligned with these construct requirements continues to increase.
Market Restraint
Propagation challenges with large or structurally complex plasmids
A major restraint arises from the difficulty laboratories encounter when working with oversized or structurally intricate plasmids. These constructs often place metabolic pressure on host cells, leading to lower transformation success and higher propagation loss during culture. Researchers must frequently adjust growth media, incubation temperatures, or antibiotic conditions to maintain plasmid integrity, increasing workflow complexity. These factors limit the speed at which teams can scale DNA-assembly pipelines and introduce delays in multi-step construct development cycles.
Market Opportunity
Expansion of collaborative strain-development programs across institutional networks
A growing opportunity is emerging from collaborative programs in which universities, biotechnology companies, and national research centres jointly develop specialised competent cell strains. These partnerships allow institutions to access tailored host systems suited for advanced applications such as metabolic pathway design, structural-biology expression studies, and large-scale library assembly. Expansion of such collaborative ecosystems increases the availability of customised strain solutions and broadens adoption across diverse research settings. As these networks grow, they are expected to elevate overall strain innovation and market accessibility at a global level.
Regional Analysis
North America held a leading position in the competent cells market in 2025 with a share of 38.34%, driven by a strong concentration of advanced molecular biology laboratories and extensive adoption of cloning workflows across academic and commercial research centers. The region benefits from coordinated programs between federal science agencies and biotechnology companies, which accelerate evaluation of new strain formats and encourage integration into genetic engineering pipelines. This environment supports rising usage of chemically competent, electrocompetent, and specialty expression strains across cloning, mutagenesis, and high-throughput screening activities.
In the U.S., market expansion is strengthened by the rapid growth of contract research facilities that manage large volumes of plasmid construction and recombinant protein projects. These facilities are incorporating strain-specific optimization protocols that reduce workflow bottlenecks and raise transformation throughput, reinforcing the country’s position as a central hub for molecular cloning services.
Asia Pacific Market Insights
Asia Pacific is progressing at a rapid pace with a CAGR of 12.66% driven by the expansion of regional genetic engineering clusters and increasing funding directed toward academic molecular biology programs. Local suppliers are scaling production of cost-focused competent cell kits, which improves accessibility for laboratories operating across urban research districts and emerging biotechnology zones. These conditions support wider deployment of cloning-ready and expression-optimized strains in research curricula, biomanufacturing training centers, and developmental biology programs.
In Japan, demand is rising due to national initiatives that promote automation-supported cloning workflows within institutional research networks. Japanese laboratories are incorporating integrated liquid-handling and controlled transformation systems that enable precise plasmid assembly operations, raising consumption of specialized strains suited for high-accuracy gene modification studies.
Regional Market share (%) in 2025
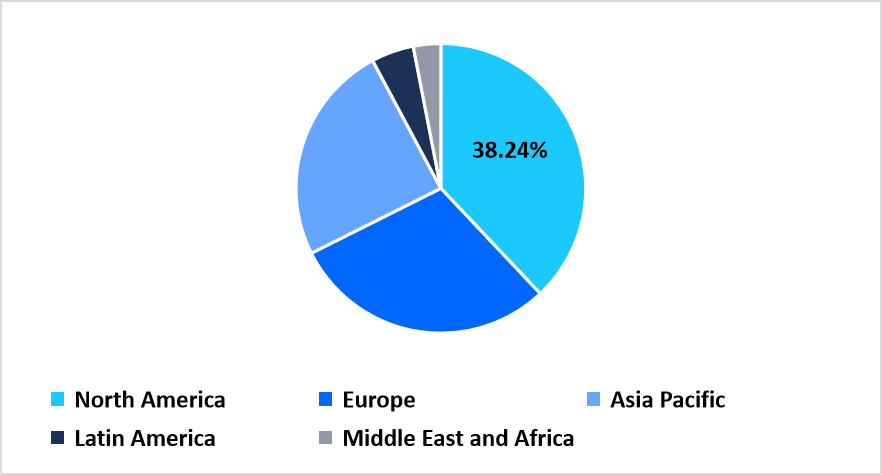
Source: Straits Research
Europe Market Insights
Europe maintains stable market growth supported by regulatory coherence across EU member states, which shortens approval pathways for molecular biology reagents. This framework encourages faster supplier expansion into research institutes working across gene modification projects, microbial engineering, and protein expression studies. Regional laboratories are adopting refined competent cell strains for multistep cloning designs and pathway reconstruction experiments.
In Germany, growth is fueled by strong collaboration between biotechnology firms and university research centers focused on strain optimization and transformation efficiency benchmarking. These partnerships generate consistent datasets that guide national purchasing decisions and stimulate ongoing adoption of high-performance cloning and expression strains across institutional networks.
Latin America Market Insights
Latin America is experiencing steady development in the competent cells market, supported by expanded procurement programs for mid-scale molecular biology labs. Increased access to compact reagent kits enables institutions in major cities to perform plasmid assembly and basic genetic modification work without reliance on large capital-intensive systems. This infrastructure model encourages broader adoption of research-grade competent strains for academic training and early-stage applied sciences.
In Argentina, demand is increasing as government-backed scientific programs emphasize laboratory skill development in genetic engineering techniques. Universities and public institutes are investing in capacity-building courses covering cloning, transformation, and plasmid handling methods, which support rising consumption of competent cells across teaching and early-career research settings.
Middle East and Africa Market Insights
The Middle East and Africa region is progressing through procurement frameworks that promote wider entry of molecular biology consumables into institutional research budgets. Subscription-based reagent access and shared-facility purchasing models enable laboratories with constrained resources to adopt competent cell technologies for introductory cloning and gene analysis projects. These structural mechanisms enhance market reach into emerging scientific ecosystems.
In South Africa, growth is driven by the expansion of genomic science programs within public universities and clinical research institutes. Dedicated molecular cloning units are being established with controlled transformation areas and specialized equipment for plasmid construction, driving increased adoption of competent cells across national academic and translational research activities.
Type Insights
The chemically competent cells segment dominated the market in 2025 with 45.67% revenue share, driven by widespread adoption across routine cloning projects and standard molecular biology workflows. Their compatibility with common transformation procedures supports frequent use in plasmid assembly, library construction, and pathway-engineering studies, positioning the segment as the leading contributor within the market.
The electrocompetent cells segment recorded the fastest growth at 11.12%, supported by rising utilisation in applications that require high transformation capacity. Increasing deployment in workflows involving large plasmids, low-copy constructs, and precision genome-editing projects accelerates the pace of expansion within this category.
Application Insights
The cloning segment dominated the market, accounting for 45.12% share in 2025. Extensive use of competent cells for subcloning, phage display library creation, toxic-DNA handling, and high-throughput construct assembly reinforces its leadership across both research and commercial settings. The segment maintains high adoption due to its broad applicability in plasmid preparation, gene assembly, and vector optimisation workflows.
The protein expression segment registered the fastest growth at 11.56%, driven by rising use of expression-ready strains in recombinant protein development and screening programs. Expanding reliance on controlled host systems for pathway studies and structural biology is contributing to accelerated uptake across institutional and industry laboratories.
End Use Insights
The pharmaceutical and biotechnology companies segment holds the dominant position in 2025 with a 42.32% share. Extensive use of competent cells in construct design, strain engineering, and development-stage molecular workflows sustains high consumption across preclinical pipelines and production-oriented laboratories.
The academic and research institutes segment grows at the fastest pace at 11.98%, supported by rising integration of molecular cloning curricula, expanded laboratory training programs, and increasing deployment of competent cells in grant-funded genetic engineering projects. The growth of interdisciplinary research centers further boosts uptake across university and national research networks.
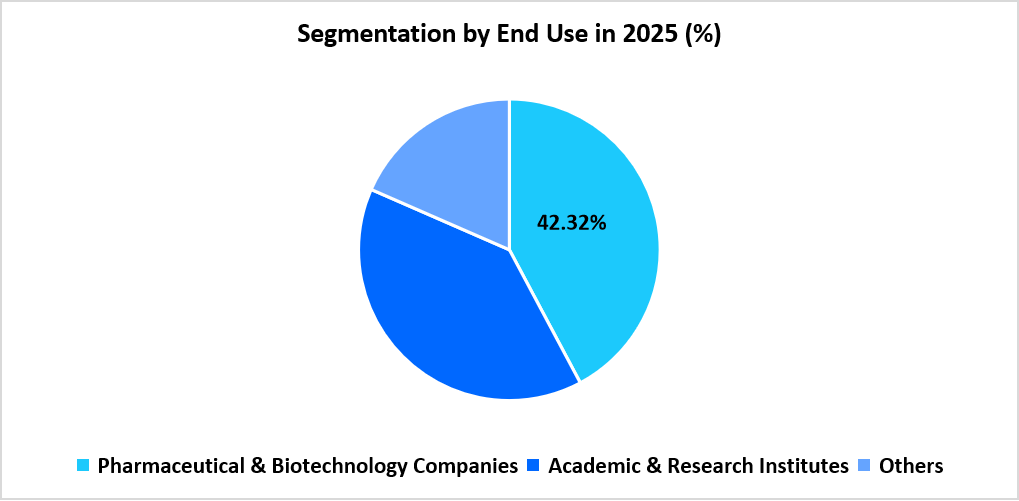
Source: Straits Research
Competitive Landscape
The global competent cells market remains moderately fragmented, with molecular biology reagent suppliers and genetic engineering solution providers maintaining leading positions. These companies expand their market presence through continuous portfolio refinement, collaborations with academic laboratories and biopharmaceutical developers, and broader distribution channels that increase availability across research, diagnostics, and industrial biotechnology settings.
New England Biolabs: An emerging market player
New England Biolabs maintains a strong role in the competent cells market with an extensive range of high-efficiency strains used in academic and commercial research. The company focuses on refining strain performance and supporting global research communities through technical partnerships and training programs. Its continued enhancements in product consistency strengthen NEB’s position across multiple application areas.
List of Key and Emerging Players in Competent Cells Market
- Thermo Fisher Scientific Inc.
- New England Biolabs
- Agilent Technologies, Inc.
- Merck KGaA
- Takara Bio Inc.
- Promega Corporation
- Bio‑Rad Laboratories, Inc.
- Qiagen
- GenScript
- OriGene Technologies, Inc.
- Zymo Research Corporation
- Illumina, Inc.
- Scarab Genomics, LLC
- HiMedia Laboratories
- Others
Strategic Initiatives
- May 2025: The Government of Gujarat, with approval from the Union government, gave the go-ahead for the state’s first Centre of Competence for Sickle Cell Anaemia (CoC).
- February 2024: U.S. researchers developed a naturally competent Vibrio natriegens platform enabling rapid, low-cost plasmid transformation. This advancement accelerated synthetic biology workflows, increasing reliance on high-performance competent cells for fast cloning, strain engineering, and scalable gene expression applications.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.86 Billion |
| Market Size in 2026 | USD 3.16 Billion |
| Market Size in 2034 | USD 7.10 Billion |
| CAGR | 10.66% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Competent Cells Market Segments
By Type
- Chemically Competent Cells
- Electrocompetent Cells
- Ultracompetent Cells
By Application
-
Cloning
- Subcloning & Routine Cloning
- Phage Display Library Construction
- Toxic/Unstable DNA Cloning
- High-throughput Cloning
- Protein Expression
-
Others
- Mutagenesis
- Single-stranded DNA Production
- Lentiviral Vector Production
- Large Plasmid Transformation
By End Use
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































