Computer-Aided Detection Market Size, Share & Trends Analysis Report By Technology (Deep Learning-Based, Machine Learning-Based, Traditional CAD Systems,, Hybrid Models, Others), By Application (Tuberculosis, Breast cancer, Lung cancer, Colon/rectal cancer, Prostate cancer, Liver cancer, Bone cancer, Others (Neurological/musculoskeletal/cardiovascular Indications)), By Indication (X-ray imaging, Computed tomography, Ultrasound imaging, Magnetic resonance imaging, Nuclear medicine imaging, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Computer-Aided Detection Market Size
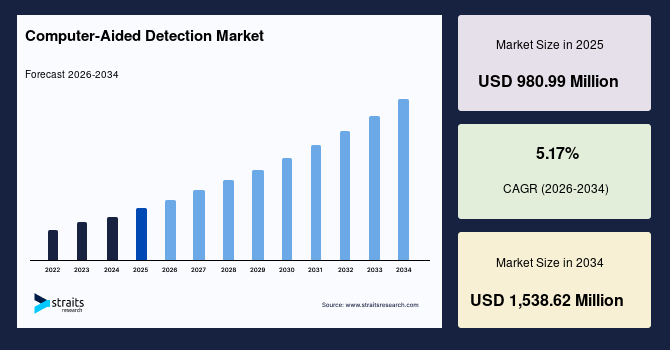
The computer-aided detection market size was valued at USD 980.99 million in 2025 and is estimated to reach USD 1,538.62 million by 2034, growing at a CAGR of 5.17% during the forecast period (2026-2034). The computer aided detection was initially used mainly to assist radiologists in clinical imaging, but it has since expanded across hospitals, diagnostic centers, and large-scale screening programs, and with the growing global emphasis on AI-driven early diagnosis and improved diagnostic accuracy, the market is poised for major growth in the coming years.
Key Market Insights
- North America dominated the computer-aided detection market with the largest share of 43.68% in 2025.
- The Asia Pacific region is expected to be the fastest-growing region in the computer aided detection market during the forecast period at a CAGR of 5.17%.
- Based on technology, the deep learning-based segment dominated the market with a revenue share of 45.23% in 2025.
- Based on application, the breast cancer segment dominated the market with a revenue share of 60.23% in 2025.
- Based on the indication, the X-ray imaging segment dominated the market with a revenue share of 35.23% in 2025.
- The US computer-aided detection market size was valued at USD 369.88 million in 2025 and is projected to reach 387.67 million in 2026.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 980.99 Million |
| Estimated 2026 Value | USD 1028.18 Million |
| Projected 2034 Value | USD 1,538.62 Million |
| CAGR (2026-2034) | 5.17% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | EDDA Technology, Inc., Olympus Corporation, Aidoc, FUJIFILM Holdings Corporation, Hologic, Inc. |
to learn more about this report Download Free Sample Report
Computer-Aided Detection Market Trends
Shift from rule-based image analysis to data-driven deep neural inference
Earlier CAD platforms relied on predefined thresholds and engineered image features, whereas current deployments increasingly rely on multi-layer neural networks trained on large-scale annotated imaging datasets. This transition supports automated detection of subtle imaging patterns across X-ray, CT, and MRI modalities and aligns CAD workflows with growing imaging volumes in hospitals and diagnostic centers.
Shift from workstation-bound CAD to cloud-enabled enterprise deployment
The market is shifting from standalone workstation-based CAD installations to cloud-enabled enterprise-wide deployment models. Vendors are increasingly designing CAD software that operates across centralized cloud platforms, enabling radiologists and clinicians to access detection outputs across multiple sites and imaging systems. This shift supports scalable deployment across health networks and improves consistency in diagnostic workflows without dependence on individual hardware configurations.
Computer-Aided Detection Market Drivers
Increasing imaging workload pressure on radiology departments drives market growth
The major driver for the computer-aided detection market is the rising pressure on radiology departments caused by growing diagnostic imaging volumes across oncology, pulmonary, and musculoskeletal care. Expansion of screening programs, aging populations, and higher utilization of CT, MRI, and X-ray imaging have increased reporting backlogs and turnaround time expectations. CAD prioritizes preliminary identification of findings in large imaging datasets, which helps radiologists manage higher case volumes amid rising disease burden globally. Thus, the market oversees a sustained adoption of CAD solutions across clinical settings such as hospitals and diagnostic imaging centers.
Growing adoption of CAD as a second-reader support tool boosts market
The computer-aided detection market is driven by the growing adoption of CAD systems as second-reader support tools. CAD does not aim to replace radiologists but as an assistance to double-check any missed readings and findings. This enhanced diagnostic confidence and reduced inter-reader variability. This approach toward consistent detection, along with human interpretation, helps standardize clinical decision-making. As a second reader, CAD flags suspicious regions and reduces callbacks, which leads to faster workaround times. In case of radiologist shortage, CAD helps in combating fatigue-related diagnostic errors. This encourages wider integration of CAD into routine diagnostic workflows.
Market Restraints
Variability in imaging data quality across clinical settings restrains adoption
Imaging data has varied quality and datasets across healthcare facilities, which results in differentiated imaging protocols and scanner configurations. This may lead to inconsistent detection and patient outcomes, as different scanner brands have different imaging hardware and scan parameters, which affect imaging data quality. Since CAD systems are trained on standardized datasets, real-world settings hamper their adoption. Inconsistent image quality leads to less accuracy and false findings, resulting in lower adoption in hospitals. This variability also results in complex model validation and limits uniform performance across diverse care settings. Thus, a heterogeneous infrastructure in the real world creates image variability and hampers athe doption of CAD systems.
Market Opportunities
Integration of CAD outputs into value-based diagnostic reimbursement models offers lucrative opportunities
An emerging market opportunity lies in integrating CAD outputs into value-based diagnostic and screening reimbursement frameworks. CAD improves early detection and accuracy with early intervention and reduced cases of missed diagnoses. It also reduces readmissions and follow-up imaging, minimizing treatment delay and maximizing patient outcomes. CAD also generates statistics related to detection sensitivity and progression metrics, enabling hospitals to report outcomes to payers and support reimbursement claims. As payers increasingly evaluate diagnostic quality and early detection outcomes, CAD systems align with performance-based payment models, offering reporting consistency and longitudinal outcome tracking. This creates opportunities for CAD vendors to position their platforms as infrastructure for outcome measurement and quality reporting rather than just pilot projects for accurate readings.
Technological Landscape
- Edison AI is a data-driven imaging AI platform that analyzes large imaging datasets and assists radiologists with detection, triage, and workflow optimization across modalities.
- AI-Rad Companion applies deep learning–based CAD algorithms to support standardized detection and quantitative assessment in CT and MRI.
- IntelliSpace AI Workflow Suite integrates CAD capabilities into enterprise imaging environments for automated image analysis, prioritization, and clinical decision support.
Regional Analysis
The computer-aided detection market in North America had a share of 43.68% in 2025. The market growth is driven by early adoption of AI-enabled medical imaging software and widespread availability of advanced diagnostic infrastructure across hospitals and imaging centers. The region benefits from a mature regulatory framework that supports clinical deployment of CAD software through structured FDA clearance pathways and strong integration with PACS and radiology information systems.
The US accounted for the largest regional share due to sustained investment in AI-based radiology tools, federal support for digital health adoption, and large-scale screening programs for cancer and cardiovascular disease. In January 2026, Cleerly disclosed on its corporate website that Aetna expanded insurance coverage for its AI-based coronary plaque analysis software, supporting broader clinical utilization of CAD-driven cardiovascular imaging in the US.
Asia Pacific
Asia Pacific is emerging as a fast-expanding region with a CAGR of 7.17% during the forecast period. The region is experiencing a surge in the number of cardiovascular cases and cancer, which leads to higher demand for early detection via CAD imaging for improved patient outcomes. Thus, a larger patient pool drives demand for CAD-assisted imaging in screening programs across hospitals. The region also has rising healthcare investments that support the deployment of AI-powered diagnostic tools. Increasing investments in CT, MRI, digital X-ray, and mammography systems also include CAD. Vendors are also collaborating with hospitals and imaging chains, as seen in the case of Aidoc’s partnerships with major hospital groups in Japan, Singapore, and Australia. AI-based chest and head CT CAD solutions are also being deployed by Qure.ai across India and Southeast Asia for screening lung diseases, strokes, and tuberculosis.
China is expected to lead the Asia Pacific computer-aided detection market during the forecast period. The market is driven by modernization programs led by the government and favorable digital health policies for improved healthcare performance. This favorable environment accelerates the deployment of AI-based imaging software, thus driving the implementation of CAD. Public healthcare institutions are also relying on CAD solutions. For instance, Tencent Miying CAD solutions are used in public hospitals for early detection of chest and oncology issues. These are also aligned with the national healthcare digitalization goals.
Europe
Europe is expected to have a strong adoption of CAD solutions across healthcare facilities, which aligns with digital imaging interoperability initiatives. The integration of AI tools in healthcare systems also aligns with regional policies of upgrading the existing systems. Unified health data exchange frameworks across the EU aim at reasonable, secure cross-border access to imaging data. This enables wider deployment of CAD tools in radiology and cardiology departments. Aidoc has deployed its AI-based CAD solutions in the region for emergency detection and improved oncology frameworks.
Germany dominates the European CAD landscape due to nationwide digital health initiatives and high installation density of advanced CT and MRI systems, which support routine use of detection algorithms in hospital diagnostics. For instance, CAD platforms integrated into radiology workflows at German university hospitals using solutions from Siemens Healthineers are actively supporting automated detection and quantification in CT and MRI imaging.
Latin America
Latin America demonstrates steady growth in the computer-aided detection market, supported by government-led digital health programs focused on strengthening diagnostic infrastructure. For example, national eHealth and digital hospital initiatives under programs such as the PAHO Digital Health Initiative promote standardized imaging data exchange, electronic health records, and AI-enabled diagnostics across public healthcare systems, creating a supportive environment for CAD deployment.
Brazil supports CAD adoption through large-scale public healthcare digitization programs. Key initiatives such as Conecte SUS and the National Telehealth Brazil Networks Program focus on integrating electronic medical records, imaging systems, and decision-support technologies, enabling public hospitals to incorporate computer-aided detection into routine diagnostic workflows.
Middle East and Africa
The Middle East and Africa computer-aided detection market is expected to have steady growth during the forecast period. The region relies on digital health transformation programs such as Vision 2030 and Digital Health Leadership that are upgrading the existing healthcare infrastructure. Increased investments in advanced diagnostic technologies focus on upgrading diagnostic imaging equipment, integrating AI into existing health facilities, expanding telehealth, and building digital health capacity. These factors lead to extensive focus on using platforms and systems that guarantee secure data exchange and patient-focused care, supporting CAD deployment for accessible imaging data.
South Africa holds a leading position in the Middle East & Africa region, owing to its focus on digitizing legacy medical records on a large scale. The region is also undertaking modernization efforts for existing radiology departments, which drives the higher adoption of CAD solutions across public hospitals. The National Department of Health of South Africa, under the national digital health framework, has introduced AI-based CAD tools in public healthcare facilities. This initiative aims at automating chest X-rays and CT scans to support screening programs for chronic issues such as tuberculosis and oncology.
Technology Insights
The deep learning-based segment dominated the computer-aided detection market share in 2025 with a share of 45.23%. With the extensive use of complex image pattern recognition across radiology and oncology workflows, CAD systems are being deployed in healthcare facilities. Deep learning models in these systems enable automated lesion detection, classification, and prioritization. This supports high-throughput diagnostic environments even in large imaging datasets.
The machine learning-based segment is projected to record a growth of 6.13% during the forecast period. Machine learning models fit well even in rule-based legacy systems, which can be deployed for structural imaging tasks. In cases where radiologists prefer statistical learning, these systems offer data building in legacy systems. These factors are expected to boost the growth of the machine learning-based segment during the forecast period.
Application Insights
The breast cancer segment held a dominant share of 60.23% in 2025, supported by the large-scale adoption of CAD software in mammography screening programs and diagnostic imaging centers. High screening volumes and standardized imaging protocols continue to drive sustained use of CAD systems in breast cancer detection workflows.
The lung cancer segment is anticipated to grow at a rate of 6.18%, driven by increasing deployment of CAD tools in chest CT screening programs and rising adoption of automated nodule detection systems in hospital radiology departments. Such factors are expected to propel the computer-aided detection market growth throughout the forecast period.
Indication Insights
The X-ray imaging segment dominated the market with a share of 35.23% in 2025, supported by widespread use of CAD solutions in chest X-ray screening, trauma assessment, and routine diagnostic imaging. High accessibility of X-ray systems across primary and secondary care facilities continues to support extensive CAD utilization.
The magnetic resonance imaging segment is expected to register a growth of 6.92%, driven by expanding use of CAD software in soft tissue visualization, oncology imaging, and neurological assessments, where advanced image interpretation supports complex diagnostic workflows.
| SEGMENT | INCLUSION | DOMINANT SEGMENT | SHARE OF DOMINANT SEGMENT, 2025 |
|---|---|---|---|
|
TECHNOLOGY |
|
Deep Learning-Based |
45.23% |
|
APPLICATION |
|
Breast cancer |
60.23% |
|
INDICATION |
|
X-ray imaging |
35.23% |
|
REGION |
|
North America |
43.68% |
Regulatory Bodies Governing Computer-Aided Detection Market
| REGULATORY BODY | COUNTRY/REGION |
|---|---|
|
US Food and Drug Administration (FDA) |
US |
|
European Medicines Agency (EMA) & National Competent Authorities (under MDR) |
Europe |
|
Health Canada (Medical Devices Directorate) |
Canada |
|
Pharmaceuticals and Medical Devices Agency (PMDA) |
Japan |
|
National Medical Products Administration (NMPA) |
China |
Competitive Landscape
The computer-aided detection market is moderately fragmented with a mix of established medical imaging companies, specialized AI diagnostic software providers, regional developers, and emerging health tech companies. Factors such as diagnostic accuracy, deployment flexibility, clinical workflow integration, diversity of training datasets, regulatory approvals, and the breadth of detectable conditions govern the competitive landscape in this market. Emerging trends in the market include machine learning integration, a shift toward cloud-based solutions, multi-condition detection, and biomarker extraction.
List of Key and Emerging Players in Computer-Aided Detection Market
- EDDA Technology, Inc.
- Olympus Corporation
- Aidoc
- FUJIFILM Holdings Corporation
- Hologic, Inc.
- Koninklijke Philips N.V.
- Siemens Healthineers AG
- NANO-X IMAGING LTD.
- Canon Medical Systems Corporation
- GE Healthcare
- Quibim
- IBM
- Riverain Technologies
- iCAD, Inc.
- Median Technologies
- Hitachi, Ltd.
- Shimadzu Analytical (India) Pvt. Ltd
- Carestream Health
- Esaote SpA
- Cleerly
- Planmeca
- DeepHealth
- AZmed
- Medical Care Technologies Inc.
Latest News on Key and Emerging Players
| TIMELINE | COMPANY | DEVELOPMENT |
|---|---|---|
|
January 2026 |
Aidoc |
The company received FDA approval for its comprehensive AI tool to detect a range of conditions from CT imaging, reinforcing its impact on CAD workflows. |
|
December 2025 |
AZmed |
The company announced CE marking for its Rayvolve LN computer-aided detection software. |
|
November 2025 |
Fujifilm India |
The company launched its AI CAD EYE endoscopy at the Fortis Hospital, Jaipur. |
|
November 2025 |
DeepHealth |
The company showcased Next-Generation Imaging Informatics and Clinical AI Solutions at the RSNA 2025. |
|
September 2025 |
GE HealthCare |
The company entered into an agreement to acquire icometrix, a developer of icobrain aria, a computer-aided detection and diagnosis tool for Alzheimer’s. |
|
September 2025 |
Aidoc |
The company received FDA Breakthrough Device Designation for its multi-triage AI solution for CT imaging. |
|
September 2025 |
Olympus Corporation |
The company launched its cloud native AI-powered suite, the OLYSENSE CAD/AI portfolio in Europe and the US. |
|
August 2025 |
GE HealthCare |
GE HealthCare collaborated with Taihao Biomedical to include its breast ultrasound AI software as a cooperative product for GE’s Invenia Automated Breast Ultrasound (ABUS) system. |
|
August 2025 |
Medical Care Technologies Inc. (MDCE) |
MDCE is an early-stage technology company that filed a US provisional patent application (No. 63/854,935) for an AI-assisted imaging system. |
|
July 2025 |
iCAD |
iCAD was acquired by DeepHealth, a subsidiary of RadNet, Inc., to expand its AI imaging capabilities. |
|
July 2025 |
Quibim |
Quibim deployed prostate cancer detection software across seven National Health Service hospitals in England. |
Source: Secondary Research
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 980.99 Million |
| Market Size in 2026 | USD 1028.18 Million |
| Market Size in 2034 | USD 1,538.62 Million |
| CAGR | 5.17% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Technology, By Application, By Indication |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Computer-Aided Detection Market Segments
By Technology
- Deep Learning-Based
- Machine Learning-Based
- Traditional CAD Systems,
- Hybrid Models
- Others
By Application
- Tuberculosis
- Breast cancer
- Lung cancer
- Colon/rectal cancer
- Prostate cancer
- Liver cancer
- Bone cancer
- Others (Neurological/musculoskeletal/cardiovascular Indications)
By Indication
- X-ray imaging
- Computed tomography
- Ultrasound imaging
- Magnetic resonance imaging
- Nuclear medicine imaging
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































