Dermatology Contract Manufacturing Market Size, Share & Trends Analysis Report By Dosage Form (Semi-Solid Formulations, Liquid Formulations, Solid Formulations, Transdermal Products, Devices / Combination Products, Others), By Service (Formulation Development, Analytical Method Development, Scale-Up & Process Validation, Commercial Manufacturing (CM), Packaging & Labelling, Others), By Indication (Acne, Psoriasis, Rosacea, Alopecia, Others), By End Use (Pharmaceutical & Biopharmaceutical Companies, Specialty Dermatology Companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Dermatology Contract Manufacturing Market Overview
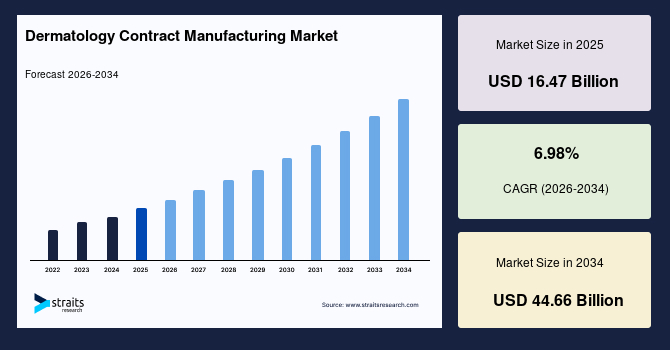
The global dermatology contract manufacturing market size is valued at USD 16.47 billion in 2025 and is estimated to reach USD 44.66 billion by 2034, growing at a CAGR of 11.76% during the forecast period. The consistent growth of the market is augmented by increasing outsourcing of topical and specialty dermatology formulations by pharmaceutical and specialty dermatology companies seeking scalable manufacturing capacity, regulatory alignment, and accelerated commercialization timelines.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 54% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.76% during the forecast period.
- Based on Dosage Form, semi-solid formulations dominated the market with a revenue share of 50.12% in 2025.
- Based on Service, the scale-up & process validation segment dominated the market with a revenue share of 50.12% in 2025.
- Based on the Indication, the acne segment dominated the market with a revenue share of 50.12% in 2025.
- Based on End Use, pharmaceutical & biopharmaceutical companies dominated the market with a revenue share of 50.12% in 2025.
- The U.S. dominates the market, valued at USD 5.02 billion in 2024 and reaching USD 5.59 billion in 2025.
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 16.47 billion
- 2034 Projected Market Size: USD 44.66 billion
- CAGR (2026-2034): 11.76%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The dermatology contract manufacturing market comprises outsourced development, manufacturing, and packaging services dedicated to pharmaceutical and specialty dermatology products intended for the treatment and management of skin-related conditions. This market supports brand owners and developers across a wide range of dosage forms, including semi-solid formulations such as creams, ointments, gels, and related formats, liquid formulations, including sprays, foams, and dermal injectables, as well as solid formulations, transdermal products, devices, or combination products, and other dermatology-specific delivery systems. Services within the market span formulation development, analytical method development, scale-up and process validation, covering contract manufacturing and clinical or batch manufacturing, commercial manufacturing, packaging and labelling, and other supporting activities. The market addresses multiple therapeutic indications, including acne, psoriasis, rosacea, alopecia, and other dermatological disorders. End-use demand is driven by pharmaceutical and biopharmaceutical companies, specialty dermatology companies, and other healthcare product developers seeking external manufacturing partners to support product development, regulatory compliance, and commercial supply across dermatology portfolios.
Latest Market Trends
Shift from standardized topical manufacturing to formulation differentiation-driven outsourcing
A defining trend in the dermatology contract manufacturing market is the shift from standardized topical production to formulation differentiation-driven outsourcing. Brand owners increasingly prioritize contract manufacturers with capabilities to tailor rheology, sensory attributes, absorption profiles, and preservative systems according to dermatological use cases. This shift reflects rising demand for customized creams, gels, foams, and injectables designed for sensitive skin, chronic inflammatory conditions, and aesthetic dermatology, positioning formulation specialization as a key outsourcing criterion.
Shift from volume-based manufacturing to lifecycle-oriented manufacturing partnerships
The notable trend is the shift from volume-based manufacturing to lifecycle-oriented manufacturing partnerships. Dermatology companies increasingly engage contract manufacturers across early development, scale-up, validation, and post-commercialization phases rather than limiting engagement to bulk production. This transition supports continuity in process knowledge, faster reformulation cycles, and improved responsiveness to regulatory updates across mature dermatology product portfolios.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 16.47 Billion |
| Estimated 2026 Value | USD 18.35 Billion |
| Projected 2034 Value | USD 44.66 Billion |
| CAGR (2026-2034) | 6.98% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Akums Drugs & Pharmaceuticals Ltd., Glamris Dermacare, Lifevision Healthcare, Allrite Group, Medconic Dermaceutics |
to learn more about this report Download Free Sample Report
Dermatology Contract Manufacturing Market Driver
Expansion of branded and specialty dermatology pipelines
The dermatology contract manufacturing market is driven by the expansion of branded and specialty dermatology pipelines across prescription, dermocosmetic, and aesthetic segments. Pharmaceutical and specialty dermatology companies increasingly launch differentiated products targeting acne, alopecia, pigmentation disorders, and inflammatory skin diseases, driving sustained demand for external partners capable of managing diverse formulation and manufacturing requirements.
Market Restraint
Complexity in managing dermatology-specific excipients and stability profiles
A key restraint in the dermatology contract manufacturing market is the complexity in managing dermatology-specific excipients and stability profiles. Semi-solid and liquid dermatology products require precise control of emulsifiers, penetration enhancers, fragrances, and active compatibility, increasing development timelines and technical risk for contract manufacturers supporting multiple client formulations.
Market Opportunity
Rising outsourcing demand from emerging specialty dermatology brands
A notable opportunity exists in rising outsourcing demand from emerging specialty dermatology brands seeking flexible manufacturing models. These companies often lack internal manufacturing infrastructure and prefer contract partners offering small to mid-scale production, rapid development support, and packaging flexibility, creating growth potential for contract manufacturers positioned toward agile and customized dermatology manufacturing services.
Regional Analysis
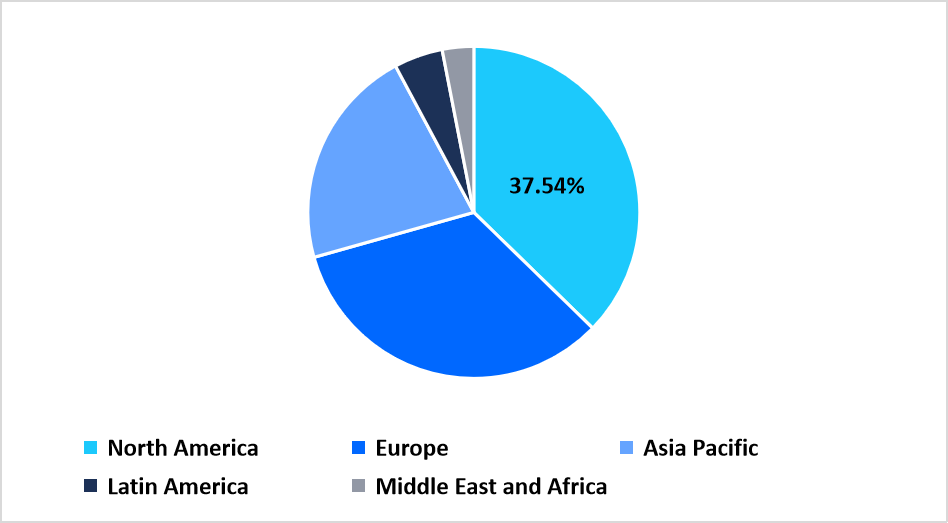
North America accounted for a leading revenue share of the dermatology contract manufacturing market in 2025, with a share of 37.54%, supported by high outsourcing penetration for prescription dermatology and cosmeceutical products. Brand owners increasingly delegate formulation development, scale-up, and commercial manufacturing to contract partners to manage regulatory complexity and accelerate product launches. Strong demand for acne, psoriasis, atopic dermatitis, and aesthetic dermatology treatments sustains continuous manufacturing engagement.
The U.S. dermatology contract manufacturing market is driven by advanced capabilities in semi-solid formulations, sterile dermatological injectables, and transdermal systems. Contract manufacturers emphasize compliance-driven operations and lifecycle manufacturing support for branded and generic dermatology portfolios.
Asia Pacific Market Insights
Asia Pacific is projected to record the fastest growth of 13.76% during the forecast period due to the rapid expansion of pharmaceutical manufacturing infrastructure and rising global outsourcing. Cost-competitive production, combined with improving regulatory alignment, attracts multinational dermatology brands seeking diversified supply chains. Regional manufacturers expand capabilities across development, primary manufacturing, and packaging.
India's dermatology contract manufacturing market growth is driven by large-scale topical production capacity and strong expertise in dermatology generics and branded formulations. Increasing exports and partnerships with global pharmaceutical companies support sustained market momentum.
Regional Market share (%) in 2025
Source: Straits Research
Europe Market Insights
Europe demonstrates steady market expansion supported by harmonized regulatory standards and high-quality manufacturing expectations for dermatological therapies. Contract manufacturers focus on precise control of excipients, preservative systems, and dermatology-specific actives to meet stringent safety benchmarks. Outsourcing activity is supported by mid-sized pharmaceutical companies seeking specialized production partners.
The French dermatology contract manufacturing market growth is driven by strong demand for dermocosmetic and prescription skin care products. Established expertise in topical formulation science and validated production processes supports long-term manufacturing collaborations.
Latin America Market Insights
Latin America reflects gradual market development with emphasis on regional manufacturing to support local dermatology brands and reduce import reliance. Contract manufacturing activity centers on formulation adaptation, secondary manufacturing, and compliance with country-specific regulatory requirements.
Mexico's dermatology contract manufacturing market growth is driven by expanding private-label dermatology production and growing domestic pharmaceutical manufacturing capabilities serving regional markets.
Middle East and Africa Market Insights
The Middle East and Africa market remains at an early stage, supported by selective investments aimed at strengthening local pharmaceutical production. Contract manufacturing activity focuses on filling, packaging, and technology transfer for established dermatology products.
United Arab Emirates dermatology contract manufacturing market growth is driven by healthcare sector expansion and initiatives supporting domestic pharmaceutical manufacturing under regional industrial development programs.
Dosage Form Insights
The semi-solid formulations segment dominated the market in 2025 with a revenue share of 50.12%. Dominance is supported by high production volumes of creams, ointments, and gels used across acne, psoriasis, and inflammatory skin disorder treatments. Strong demand for topical delivery systems with controlled absorption and patient-friendly application sustains the continuous outsourcing of semi-solid manufacturing.
The liquid formulations segment is projected to register the fastest growth with a share of 12.23%. Growth is driven by rising adoption of sprays, foams, and dermal injectables for targeted dermatological applications, supported by expanding product differentiation strategies among dermatology brands.
Service Insights
The scale-up and process validation segment dominated the market in 2025, accounting for a revenue share of 33.67%. Dominance reflects growing reliance on contract manufacturers to manage process reproducibility, batch consistency, and regulatory validation during the transition from development to commercial production.
The formulation development segment is anticipated to witness the fastest growth with a share of 12.45%. Expansion is driven by increasing demand for customized dermatology formulations addressing stability, sensory attributes, and compatibility with dermatological actives.
Indication Insights
The acne segment dominated the market in 2025 with a revenue share of 42.35%. High prevalence of acne across adolescent and adult populations supports sustained manufacturing demand for prescription and over-the-counter dermatology products.
The alopecia segment is expected to grow at the fastest rate, with a share of 12.78%. Growth is supported by rising clinical focus on hair loss treatments and increasing commercialization of topical and injectable alopecia therapies.
End Use Insights
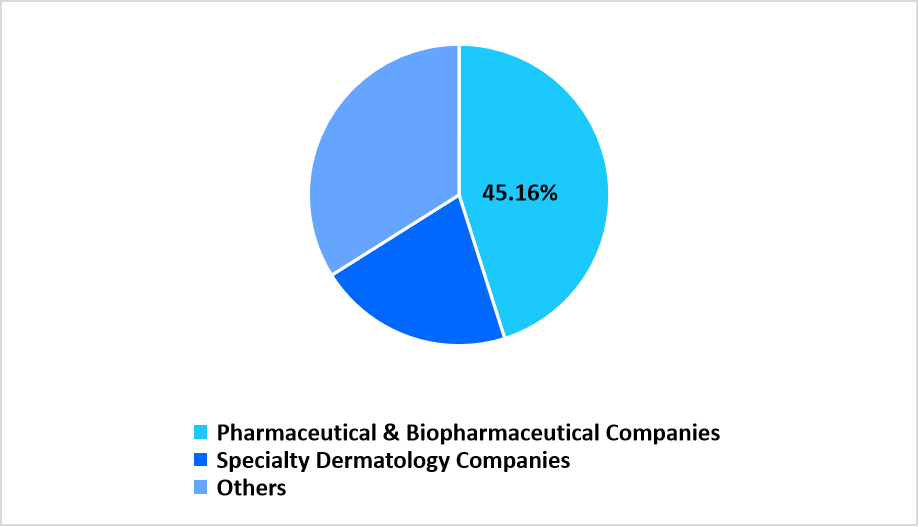
The pharmaceutical and biopharmaceutical companies segment dominated the market in 2025, representing a revenue share of 45.16%. Strong outsourcing intensity and broad dermatology product portfolios drive continued reliance on contract manufacturing partners.
The specialty dermatology companies segment is projected to register the fastest growth with a share of 12.99%. Expansion is driven by the increasing emergence of focused dermatology brands seeking flexible manufacturing models and rapid product commercialization pathways.
By End Use Market Share (%), 2025
Source: Straits Research
Competitive Landscape
The global dermatology contract manufacturing market is moderately fragmented, with a mix of large pharmaceutical service providers and regionally specialized manufacturers offering formulation development, commercial manufacturing, and packaging services. Competition centers on topical formulation expertise, regulatory readiness, production scalability, and the ability to support diversified dermatology portfolios across prescription and consumer segments. Market participants prioritize long-term supply agreements, quality management systems, and flexible manufacturing models to serve evolving brand requirements.
Akums Drugs & Pharmaceuticals Ltd.: An emerging market player
Akums Drugs & Pharmaceuticals Ltd. is recognized as an emerging market player in the dermatology contract manufacturing market due to its expanding dermatology-focused manufacturing capabilities. The company supports a wide range of topical dosage forms, including creams, gels, lotions, ointments, and dermatology-specific combinations. Its contract manufacturing model emphasizes scalability, regulatory compliance, and support for both branded and generic dermatology products. Growing partnerships with domestic and international pharmaceutical companies, along with continued investments in manufacturing capacity and formulation development, strengthen its positioning within the emerging tier of dermatology-focused contract manufacturers.
List of Key and Emerging Players in Dermatology Contract Manufacturing Market
- Akums Drugs & Pharmaceuticals Ltd.
- Glamris Dermacare
- Lifevision Healthcare
- Allrite Group
- Medconic Dermaceutics
- Shinom Cosmeceuticals
- WISDERM
- Marvex Pharma
- Servocare Lifesciences
- Zestwin Lifesciences
- Cledral Life Sciences
- Cosmenova
- Hanisan Healthcare
- Thea Janus
- Genesis Biotec
- Others
Strategic Initiatives
- October 2024: Emcure announced the launch of a new wholly owned subsidiary, Emcutix Biopharmaceuticals, which concentrates on both therapeutic and aesthetic dermatology, reflecting the company’s strategic move to strengthen its presence and innovation capabilities within the skin health domain.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 16.47 Billion |
| Market Size in 2026 | USD 18.35 Billion |
| Market Size in 2034 | USD 44.66 Billion |
| CAGR | 6.98% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Dosage Form, By Service, By Indication, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Dermatology Contract Manufacturing Market Segments
By Dosage Form
-
Semi-Solid Formulations
- Creams
- Ointments
- Gel
- Others
-
Liquid Formulations
- Sprays
- Foams
- Dermal Injectables
- Others
- Solid Formulations
- Transdermal Products
- Devices / Combination Products
- Others
By Service
- Formulation Development
- Analytical Method Development
-
Scale-Up & Process Validation
- Contract Manufacturing
- Clinical / Batch Manufacturing
- Commercial Manufacturing (CM)
- Packaging & Labelling
- Others
By Indication
- Acne
- Psoriasis
- Rosacea
- Alopecia
- Others
By End Use
- Pharmaceutical & Biopharmaceutical Companies
- Specialty Dermatology Companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































