Gene Vector Market Size, Share & Trends Analysis Report By Type (Viral Vectors, Non-Viral Vectors), By Application (Gene Therapy, Vaccinology, Others), By Diseases (Oncology, Genetic Disorders, Infectious Diseases, Others), By End Use (Pharmaceutical and Biotechnology Companies, Academic & Research Institutes, CROs, CDMOs) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Gene Vector Market Overview
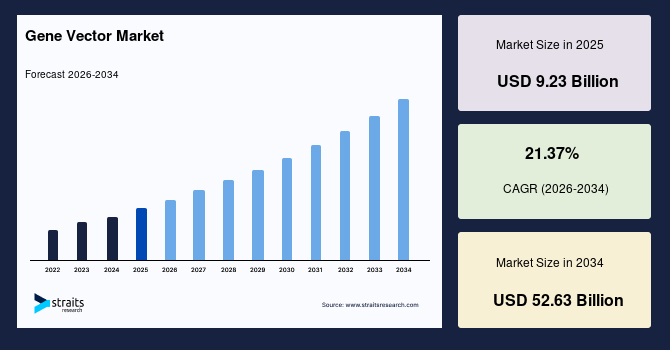
The global gene vector market size is estimated at USD 9.23 billion in 2025 and is projected to reach USD 52.63 billion by 2034, growing at a CAGR of 21.37% during the forecast period. Sustained growth of the market is propelled by the rising demand for gene therapy and advanced drug delivery systems, increasing prevalence of genetic and rare diseases, and continuous technological advancements in vector engineering and manufacturing.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 44.17%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 23.27%.
- Type: The non-viral vectors is anticipated to register the fastest CAGR of 22.12% during the forecast period.
- Application: The gene therapy segment dominated the market in 2025, with a revenue share of 35.62%.
- Disease: The oncology segment dominated the market in 2025 with a revenue share of 45.62%.
- End-User: The pharmaceutical and biotechnology companies segment dominated the market in 2025, with a revenue share of 60.02%.
- The U.S. dominates the global market, valued at USD 3.05 billion in 2024 and reaching USD 3.69 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
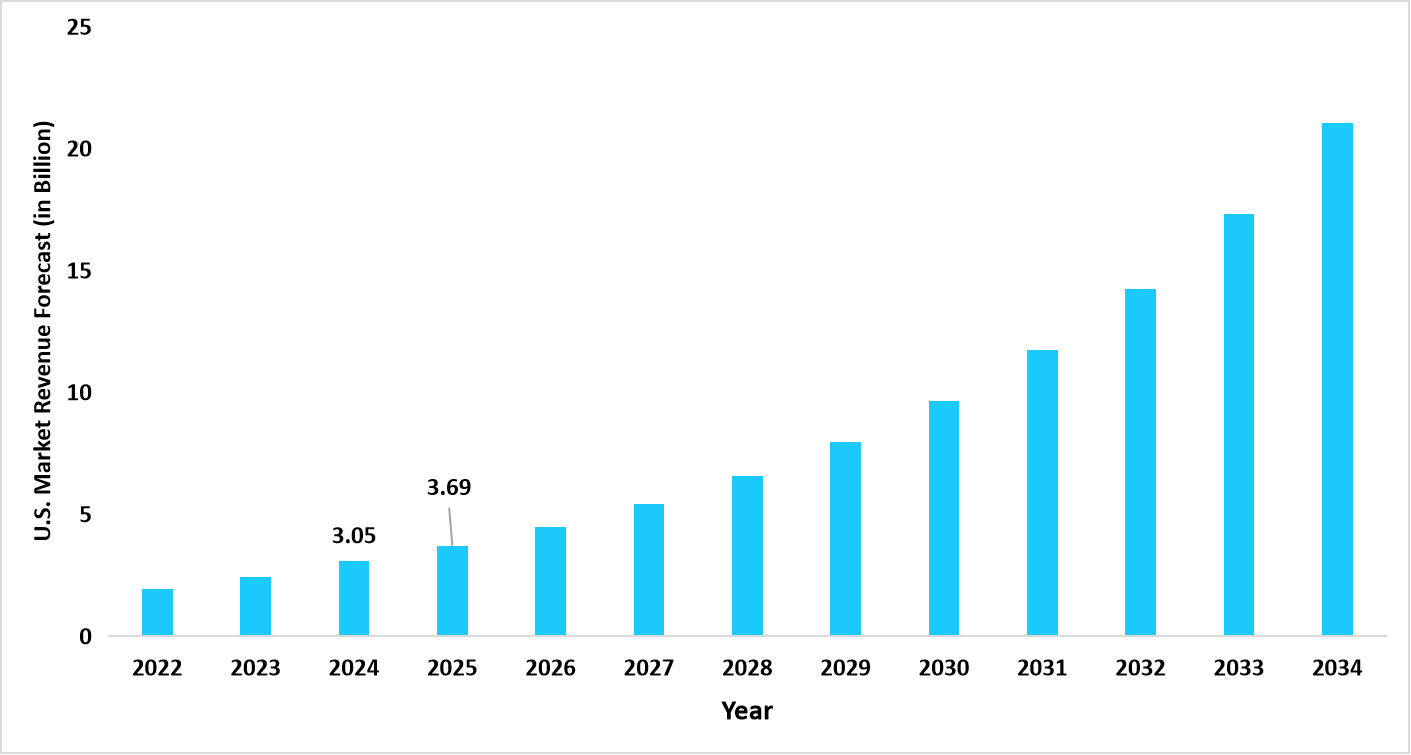
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 9.23 billion
- 2034 Projected Market Size: USD 52.63 billion
- CAGR (2025 to 2034): 21.37%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The global gene vector market refers to the industry focused on the development, manufacturing, and utilisation of delivery systems that transport therapeutic genetic material into target cells to treat or prevent diseases. Gene vectors are fundamental tools in gene therapy, vaccinology, and biomedical research, enabling precise delivery of DNA or RNA to specific tissues. These vectors are primarily classified into viral vectors, such as adenoviral, adeno-associated viral (AAV), lentiviral, retroviral, and herpes simplex virus (HSV) vectors and non-viral vectors, including plasmid DNA, lipid nanoparticles (LNPs), and polymer-based carriers. They play a pivotal role in advancing personalised and targeted treatments for oncology, genetic, and infectious diseases, with key end users comprising pharmaceutical and biotechnology companies, academic and research institutes, CROs, and CDMOs. Fueled by continuous advancements in vector engineering, increasing approvals of gene therapies, and expanding investment in regenerative medicine, the global market serves as a cornerstone of modern biotechnology, driving the evolution of next-generation therapeutic solutions.
Latest Market Trends
Shift from Viral to Non-Viral and Hybrid Gene Delivery Systems
The gene vector market is witnessing a shift from traditional viral vectors, such as adenoviral and lentiviral systems, to advanced non viral and hybrid gene delivery platforms. This transition is driven by the need to overcome safety concerns, immunogenicity, and scalability challenges associated with viral vectors. Non viral systems, including lipid nanoparticles (LNPs), polymer-based carriers, and exosome-mediated delivery, are gaining traction due to their improved biocompatibility, flexible payload capacity, and ease of large-scale manufacturing. For instance, in January 2025, Moderna and Metagenomi collaborated to develop a hybrid LNP based gene delivery platform integrating CRISPR editing tools, which highlighted the market’s evolution toward safer, more versatile, and advanced gene delivery technologies.
Integration of Artificial Intelligence and Computational Modeling in Vector Design
A major trend in the gene vector market is the integration of artificial intelligence (AI) and computational modeling to accelerate vector discovery, optimize payload design, and predict immune responses. AI-driven algorithms are being increasingly employed to analyze large genomic datasets and simulate vector host interactions, enabling the creation of more precise and efficient gene delivery systems. For example, in April 2025, Harvard’s Wyss Institute introduced an AI guided platform capable of predicting optimal AAV capsid variants for targeted tissue delivery, which further supports in reducing experimental timelines. This integration of digital technologies is transforming vector development from trial and error experimentation to data driven precision engineering, marking a new era of intelligent and predictive gene therapy design.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 9.23 Billion |
| Estimated 2026 Value | USD 11.18 Billion |
| Projected 2034 Value | USD 52.63 Billion |
| CAGR (2026-2034) | 21.37% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Lonza, Thermo Fisher Scientific Inc., Catalent, Inc, Charles River Laboratories, WuXi AppTec |
to learn more about this report Download Free Sample Report
Gene Vector Market Driver
Advancements in Vector Engineering and Delivery Technologies Driving Market Growth
A major driver for the gene vector market is the rapid advancement in vector engineering and delivery technologies, which is enhancing the safety, efficiency, and targeting precision of gene therapies. For instance, in March 2025, Regenxbio announced the development of an improved AAV based delivery platform capable of achieving higher transgene expression with reduced immunogenicity, which further improves therapeutic outcomes in retinal and neuromuscular disorders. Such innovations in vector design, capsid modification, and non-viral delivery systems transformed the landscape of gene therapy by enabling more efficient and durable treatments, thereby propelling the overall growth of the global market.
Market Restraint
High Manufacturing Complexity and Regulatory Challenges Restricting the Growth of the Market
A key restraint for the gene vector market is the high complexity of vector manufacturing and stringent regulatory requirements. Producing viral vectors such as AAV and lentivirus demands advanced facilities and strict quality controls, making large scale production costly and time consuming. Various biotech firms reported delays in gene therapy trials due to manufacturing bottlenecks and compliance challenges with evolving FDA and EMA guidelines, hindering timely product commercialization.
Market Opportunity
Expansion of Targeted and Personalized Therapies Offering Major Growth Opportunities for the Market
A major opportunity for the gene vector market arises from the growing demand for targeted and personalized therapies enabled by advancements in vector engineering and genomic medicine. Modern gene vectors, such as adeno-associated viral (AAV), lentiviral, and non-viral delivery systems, are increasingly being tailored to deliver therapeutic genes with high precision and minimal off-target effects. Several biotechnology companies partnered with genomic editing firms to develop next-generation viral vectors capable of tissue specific targeting and controlled gene expression for rare genetic and oncological disorders. This shift towards precision medicine, supported by regulatory approvals and rising investments in gene therapy R&D, is unlocking new opportunities for manufacturers to create safer, more efficient, and patient-specific vector platforms, thereby accelerating the expansion of the global market.
Regional Analysis
North America region dominated the market in 2025, with a revenue share 44.17%. The driving factor for the North America gene vector market is the increasing adoption of modular and automated viral vector manufacturing platforms by biopharmaceutical companies and CDMOs. These advanced platforms enable flexible, small batch production for personalized therapies and accelerate scalability from clinical to commercial stages. This technological shift is particularly crucial in the U.S., where the growing pipeline of AAV and lentiviral based therapies demands rapid, high quality vector production to meet stringent FDA regulatory standards and clinical trial timelines.
The rising collaboration between academic research institutions and biotech firms to develop novel, next generation vector platforms with enhanced targeting and reduced immunogenicity, enhances the market growth. U.S. universities and federal research centers are increasingly partnering with industry players to translate early stage vector innovations, such as engineered AAV capsids and non-viral delivery systems into clinically viable products. This strong academia industry synergy fosters rapid innovation, access to advanced R&D infrastructure, and a steady pipeline of proprietary vector technologies driving market growth.
Asia Pacific Gene Vector Insights
Asia Pacific region is anticipated to register the fastest CAGR of 23.27% during the forecast period. The rapid establishment of regional biomanufacturing hubs supported by government led biotechnology initiatives, enhances the market growth. Countries such as China, South Korea, and Singapore are investing heavily in building advanced viral vector production facilities and offering incentives to attract international biotech firms.
The growing focus on indigenous R&D and public private partnerships to advance affordable gene therapy solutions, drives the market growth. Indian biotech firms and research institutes are increasingly investing in developing cost efficient viral and non-viral vector systems tailored to local disease burdens, such as genetic blood disorders and rare metabolic conditions. Supported by initiatives like “Make in India” and the Biotechnology Industry Research Assistance Council (BIRAC), these collaborations are fostering domestic innovation, technology transfer, and scalable vector manufacturing capabilities, positioning India as an emerging hub for low-cost gene therapy development.
By Region Market Share (in percent share %), 2025
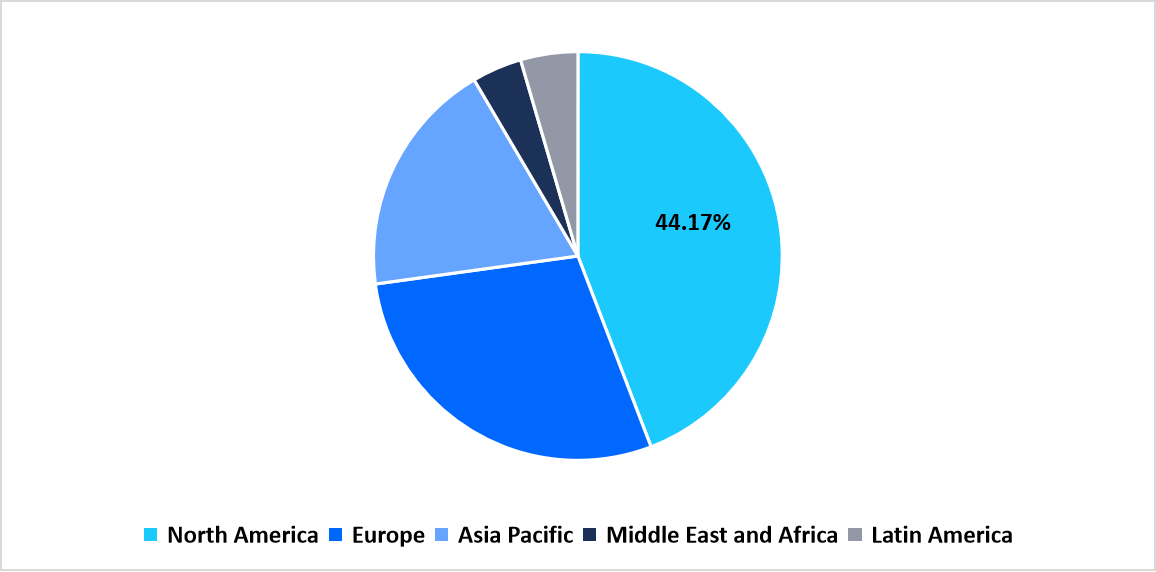
Source: Straits Research
Europe Gene Vector Insights
The strong regulatory and ethical framework promoting the development of safe and standardized vector based therapies, drives the market growth. The European Medicines Agency (EMA) implemented specialized guidelines for advanced therapy medicinal products (ATMPs), ensuring high quality, reproducible, and ethically compliant vector production. This potent regulatory environment encouraged innovation while maintaining patient safety, attracting both established pharmaceutical companies and startups to conduct gene therapy research and manufacturing within Europe.
The growth of dedicated manufacturing hubs like the Cell and Gene Therapy Catapult, which provide GMP facilities, technical expertise, and regulatory support, drives the market growth. These initiatives, backed by government funding, are accelerating scalable and high quality vector production across the country, which further enhances the market growth.
Latin America Gene Vector Insights
The rising government investment in genomic medicine and rare disease research, enhances the market growth. National health programs in countries such as Brazil, Argentina, and Chile are promoting the use of advanced genetic tools, creating demand for efficient viral and non viral vectors. This policy driven focus on precision medicine is encouraging local biotech startups and academic institutions to develop region specific vector technologies and manufacturing capabilities.
The emergence of biotechnology incubators and academic spin offs focused on localized vector innovation, drives the market growth. Supported by national science and technology grants, Argentine universities and research centers developed homegrown gene delivery platforms to address regional genetic disorders. This growing ecosystem of innovation is fostering self reliance, enhancing domestic manufacturing capacity, and attracting international collaborations in the gene vector space.
Middle East and Africa Gene Vector Insights
The growing influx of international biotech partnerships and technology transfer agreements, drives the market growth. Global pharmaceutical companies collaborated with regional healthcare institutions to establish localized gene therapy and vector production capabilities, which further drives the market growth. These alliances enhanced technical expertise, reduced import dependency, and accelerated the adoption of advanced gene delivery technologies across the region.
The development of advanced biomedical research infrastructure supported by government and international funding, drives the market growth. Institutions such as the Council for Scientific and Industrial Research (CSIR) and the South African Medical Research Council (SAMRC) invested in molecular biology and gene therapy platforms, enabling local R&D in viral vector production. This growing research capacity is positioning South Africa as a regional hub for genetic innovation and clinical translation in sub-Saharan Africa.
Type Insights
The viral vectors segment dominated the market in 2025, owing to their high gene delivery efficiency, stable gene expression, and broad applicability across gene therapy and vaccine development. Their proven clinical performance, coupled with the rising number of FDA and EMA approvals for AAV- and lentiviral-based therapies, further strengthened their adoption.
The non viral vectors segment is anticipated to register the fastest CAGR of 22.12% during the forecast period, due to the increasing demand for safer and cost-effective gene delivery alternatives. These vectors eliminate the risk of insertional mutagenesis and immune response associated with viral systems, making them suitable for repeated administration.
Application Insights
The gene therapy segment dominated the market in 2025, with a revenue share of 35.62%, due to the growing number of clinical trials and regulatory approvals for vector-based gene therapies targeting rare and inherited diseases.
The vaccinology is anticipated to register the fastest CAGR of 22.34% during the forecast period, due to the growing adoption of viral vectors in developing vaccines for infectious and emerging diseases. The success of adenoviral and AAV-based vaccine platforms during the COVID-19 pandemic demonstrated their scalability, safety, and rapid development potential.
By Application Market Share (in percent share %), 2025
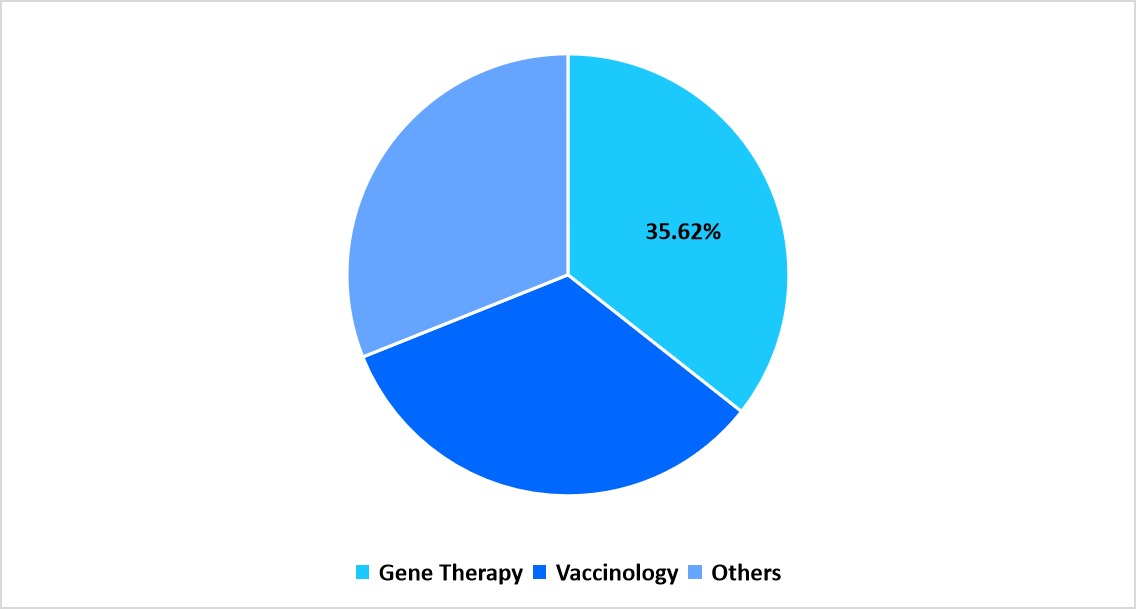
Source: Straits Research
Disease Insights
The oncology segment dominated the market in 2025, with a revenue share of 45.62%. The growth is attributed to the extensive use of viral and non-viral vectors in cancer gene therapy, oncolytic virotherapy, and vaccine development. The rising incidence of cancer worldwide, coupled with growing research in targeted and personalized treatments, has increased demand for efficient gene delivery systems.
The genetic disorders segment is anticipated to register the fastest CAGR of 22.74% during the forecast period, driven by the increasing approval of gene therapies targeting rare and inherited diseases such as spinal muscular atrophy (SMA), haemophilia, and Duchenne muscular dystrophy. Advances in vector design, improved safety profiles, and strong regulatory and funding support for rare disease research are accelerating the adoption of gene vector based therapies in this segment.
End–Use Insights
The pharmaceutical and biotechnology companies segment dominated the global gene vector market in 2025 with a revenue share of 60.02%, owing to the rising number of in house gene therapy programs and large scale investments in vector manufacturing capabilities.
The CDMOs segment is anticipated to register the fastest CAGR of 22.89% during the forecast period, driven by the growing outsourcing of vector production by small and mid sized biotech firms lacking in house manufacturing capacity.
Competitive Landscape
The global gene vector market is moderately fragmented, consisting of a mix of well-established biopharmaceutical companies and innovative biotechnology startups focusing on advanced vector design, scalable manufacturing, and regulatory compliance.
Vivet Therapeutics:An emerging market player
Vivet Therapeutics, a France based biotechnology company, developed VTX-801, an adeno associated virus (AAV) based gene therapy vector designed for the treatment of Wilson’s disease. The company’s proprietary vector optimization and delivery platform enabled efficient liver targeted gene transfer and long term transgene expression, positioning it as a promising innovator in the gene vector domain.
List of Key and Emerging Players in Gene Vector Market
- Lonza
- Thermo Fisher Scientific Inc.
- Catalent, Inc
- Charles River Laboratories
- WuXi AppTec
- Oxford Biomedica PLC
- Sartorius AG
- Takara Bio Inc.
- uniQure NV.
- Pfizer Inc.
- bluebird bio, Inc.
- Boehringer Ingelheim International GmbH
- Cytiva
- Revvity
- Forge Biologics
- GENE VECTOR BARCELONA S.L.
- Miltenyi Biotec
- Astellas Pharma Inc.
- Dyno
- Hoffmann-La Roche Ltd
- Others
Strategic Initiatives
- October 2025: Researchers at Johns Hopkins University developed a targeted adeno-associated virus (AAV) gene therapy vector for Neurofibromatosis Type 1 (NF1) tumors, which delivered a functional NF1 gene fragment with improved tumor targeting and showed reduced tumor growth in preclinical studies.
- October 2025: Australia launched its first clinical and commercial scale viral vector manufacturing facility, the Viral Vector Manufacturing Facility (VVMF) in Sydney’s Westmead Health & Innovation Precinct, with an aim of producing GMP grade lentiviral (LV) and AAV vectors for gene and cell therapies, spanning R&D, construct design, process development and up to 500 L clinical/ commercial manufacturing.
- October 2025: A team at Tel Aviv University developed a self-complementary AAV (scAAV) gene therapy vector targeting mutations in the CLIC5 gene, successfully preventing hair cell degeneration and preserving hearing and balance in animal models of hereditary inner ear dysfunction.
- October 2025: Opus Genetics announced that their gene therapy candidate OPGxLCA5, designed to treat inherited retinal degeneration caused by biallelic mutations in the LCA5 gene, achieved 12-month positive results in adult patients, including sustained improvements in vision and functional mobility and the company announced that the first pediatric patient had shown meaningful visual improvement one month after treatment.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 9.23 Billion |
| Market Size in 2026 | USD 11.18 Billion |
| Market Size in 2034 | USD 52.63 Billion |
| CAGR | 21.37% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By Diseases, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Gene Vector Market Segments
By Type
-
Viral Vectors
- Adenoviral vectors
- Adeno associated viral vectors
- Retroviral vectors
- Lentiviral vectors
- Herpes Simplex Virus (HSV) Vectors
- Others
-
Non-Viral Vectors
- Plasmid DNA
- Lipid Nanoparticles
- Polymer-based Vectors
- Others
By Application
- Gene Therapy
- Vaccinology
- Others
By Diseases
- Oncology
- Genetic Disorders
- Infectious Diseases
- Others
By End Use
- Pharmaceutical and Biotechnology Companies
- Academic & Research Institutes
- CROs
- CDMOs
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.























