Gold Nanoparticles Market Size, Share & Trends Analysis Report By Type (Water-soluble AuNPs, Oil-soluble AuNPs, Dual-phase (water + oil) soluble AuNPs), By Application (Imaging, Targeted drug delivery, Sensors, Invitro diagnostics, Probes, Catalysis, Others), By End-User Industry (Healthcare & Pharmaceuticals, Electronics & Electrical, Chemicals, Others (Cosmetics, Personal Care, Glass, Photometry)) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Gold Nanoparticles Market Overview
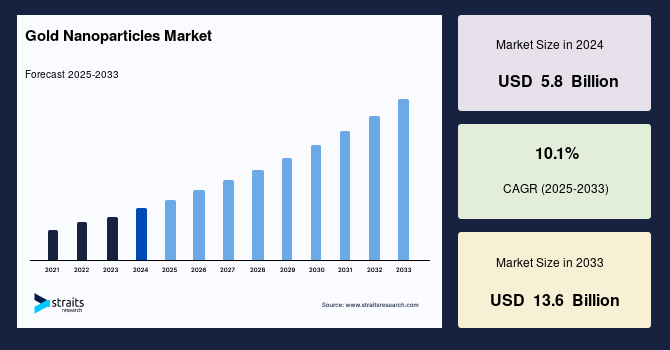
The global gold nanoparticles market size was valued at USD 5.8 billion in 2024 and is projected to reach from USD 6.4 billion in 2025 to USD 13.6 billion by 2033, growing at a CAGR of 10.1% during the forecast period (2025-2033). The global market is experiencing strong growth, driven by its unique optical, electronic, and biochemical properties. Key growth drivers include expanding applications in biomedicine, i.e., targeted drug delivery, imaging, diagnostics, and electronics like conductive inks and nanosensors. Recent advances in scalable, cost-effective synthesis methods make high-quality nanoparticles more accessible.
Key Market Insights
- North America dominated the gold nanoparticles industry and accounted for a 26.7% share in 2024
- Based on application, the imaging segment leads the market with the largest revenue share of 21% in 2024.
- Based on type, water-soluble gold nanoparticles dominate the market
Market Size & Forecast
- 2024 Market Size: USD 1.71 Billion
- 2033 Projected Market Size: USD 5.02 Billion
- CAGR (2025-2033): 10.1%
- Largest market in 2024: North America
- Fastest growing market: Asia Pacific
Additionally, the trend toward miniaturisation in electronics and personalised medicine is increasing demand for nanoparticles with precise size and surface chemistry. Asia-Pacific is emerging as the fastest-growing region due to robust investments in healthcare, pharmaceutical research, and electronics manufacturing. Meanwhile, North America and Europe continue to lead in R&D intensity, regulatory frameworks, and advanced nanotech adoption. As synthesis methods improve and applications expand, the market is poised for sustainable double-digit growth through the forecast period.
Market Trend
Rapid Innovation in Synthesis and Functionalisation Techniques
A prominent trend is the rapid innovation in synthesis and functionalisation techniques, aimed at producing highly uniform, biocompatible gold nanoparticles at scale. Leading companies are filing patents for greener, plant- or microbial-mediated “biosynthesis” approaches that avoid harsh chemicals while ensuring precise size control.
- For instance, research from Goa University in February 2024 demonstrated mushroom-derived gold nanoparticles with enhanced anti-cancer activity, showcased in a peer-reviewed study. Industrial labs are investing heavily in continuous-flow reactors and microfluidics, which improve reproducibility and reduce batch variation, a crucial factor for medical-grade production.
This trend is also extending into electronics. Nanoparticles tailored for conductive inks and printed electronics are emerging, supported by their stable plasmonic behaviour and ease of functionalisation. The development of size- and shape-controlled particles enables plasmonic resonance tuning, improving sensor sensitivity and electronic functionality. As next-gen CMOS and photonic devices drive nanomaterial requirements, this trend ensures that gold nanoparticles remain central to cutting-edge innovation in healthcare and electronics.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 5.8 Billion |
| Estimated 2025 Value | USD 6.4 Billion |
| Projected 2033 Value | USD 13.6 Billion |
| CAGR (2025-2033) | 10.1% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
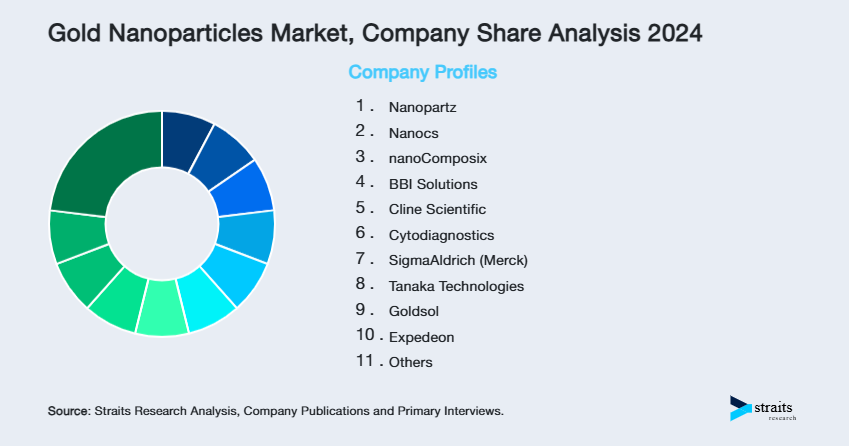
| Key Market Players | Nanopartz, Nanocs, nanoComposix, BBI Solutions, Cline Scientific |
to learn more about this report Download Free Sample Report
Market Driver
Demand in Biomedical Applications
The key driver fueling growth is the explosive demand in biomedical applications, especially targeted drug delivery, diagnostics, imaging, and cancer therapies. Gold nanoparticles’ biocompatibility, tunable surface chemistry, and plasmonic properties make them ideal for precision medicine. The use of gold nanoparticles as radiosensitizers in proton therapy is garnering attention as nanoparticles significantly enhance tumour targeting and treatment efficacy.
- For instance, in April 2025, Drug Target Review spotlighted Gylden Pharma’s novel gold-nanoparticle vaccine platform, which trains T cells to recognise and destroy infected cells, a major leap forward in cellular immunity
Major pharmaceutical players like Gilead Sciences and GSK are actively incorporating gold nanoparticles into vaccines and diagnostic kits. Beyond oncology, applications in antimicrobial coatings, biosensors, and theranostics are gaining traction, driving healthcare expenditures and research funding.
Market Restraint
High Production Costs and Scalability Challenges
Despite strong growth, high production costs and scalability challenges remain significant restraints. Precision control over size, shape, and surface functionalisation often entails expensive materials, energy-intensive processes, and stringent lab conditions. Current production techniques, including chemical reduction, laser ablation, and biological synthesis, exhibit significant limitations regarding yield consistency, reproducibility, and cost-efficiency when scaled to industrial levels. Transitioning from laboratory-scale synthesis to full-scale manufacturing demands substantial capital expenditure in specialised infrastructure such as cleanrooms, microfluidic platforms, and continuous-flow reactors, elevating unit costs considerably.
This financial burden restricts market penetration among small and medium-scale enterprises in price-sensitive regions, particularly across emerging markets outside North America and Europe. Additionally, the complex regulatory frameworks surrounding nanoparticle safety, toxicity profiling, and environmental impact assessments impose extended timelines and increased compliance costs, further compounding the scalability dilemma for manufacturers.
Market Opportunity
Integration with Next-Generation Therapies and Diagnostics
The integration of gold nanoparticles (GNPs) into emerging therapeutic and diagnostic modalities represents a significant opportunity for the global market. In oncology, GNPs are highly effective radiosensitizers, particularly in proton therapy, enhancing tumour targeting precision while minimising damage to surrounding healthy tissue. Similarly, the convergence of nanotechnology with AI-powered biosensing platforms and theranostics fosters the development of highly sensitive, rapid diagnostic systems capable of real-time disease detection at point-of-care settings.
- For example, in May 2025, a team led by Dr. Sandeepta Burgula at Osmania University in Hyderabad unveiled a 30-minute, low‑cost sepsis diagnostic leveraging gold nanoparticles: a nitrocellulose strip-based test offering up to 90% accuracy. This development shows integrating gold nanoparticles into next-generation diagnostics, especially in emerging markets
Moreover, coupled with rising R&D investments, particularly in Asia-Pacific and North America, these technological synergies position gold nanoparticles as indispensable assets in the evolution of precision healthcare and advanced electronic applications.
Regional Analysis
North America accounted for the largest share of 26.7% in the gold nanoparticles industry in 2024, thanks to its mature healthcare infrastructure, strong R&D ecosystem, and early adoption of nanotech in medicine and electronics. The U.S. alone houses numerous clinical trials exploring AuNPs in proton therapy radiosensitisation, cancer imaging, and precision drug delivery. Major electronics firms like Intel and Samsung are integrating AuNPs in conductive inks, sensors, and wearable devices, responding to the IoT and 5G boom.
The region's favourable regulatory environment, including fast-track FDA approvals for nanomedicine, aids commercialisation. The electronics sector complements healthcare contract manufacturers, who leverage AuNP conductive inks for printed sensors. North America’s robust ecosystem, comprising well-funded healthcare and electronics industries, proactive regulation, and strong public-private R&D partnerships, positions it as the dominant regional market.
The U.S. remains the global leader in the gold nanoparticles (AuNPs) market, driven by its strong healthcare, biotechnology, and electronics industries. AuNPs are widely applied in drug delivery, diagnostics, biosensing, and nanomedicine, with multiple ongoing FDA-backed clinical trials in oncology and precision diagnostics. The National Nanotechnology Initiative (NNI) received over USD 2.2 billion in funding for FY 2025, directly supporting nanoscale innovation in the biomedical and microelectronics fields. Private sector involvement is robust, with increasing venture capital investments targeting green synthesis and advanced nanotherapies. This blend of public funding, commercial R&D, and regulatory support ensures that the U.S. will continue leading the commercialisation and innovation of AuNP technologies through the forecast period.
Canada is emerging as a strong player in the AuNP space, particularly within in vitro diagnostics and biosensing. Companies such as Cytodiagnostics and Luminex are developing innovative nanoparticle-based assays for early cancer detection and infectious disease monitoring. Federal and provincial funding supports projects targeting cancer diagnostics, biosensors, and green nanoparticle synthesis methods. Strong collaborations between private companies have yielded several breakthroughs in eco-friendly synthesis and scalable production. Canada’s growing focus on sustainability, clinical research infrastructure, and public-private partnerships allows it to rapidly narrow the innovation gap with the U.S., creating a thriving ecosystem for nanoparticle-enabled diagnostics and therapies.
Asia Pacific Market Trends
Asia-Pacific (APAC) is witnessing the fastest growth in the global gold nanoparticles market, driven by rapid industrialisation, government support, and rising healthcare demands across China, India, Japan, and South Korea. The region’s growth trajectory benefits from its ability to scale manufacturing while advancing high-end clinical and electronic applications. The region’s booming electronics sector adds to growth.
Japan's NTT and Panasonic are co-developing AuNP conductive inks for flexible displays and environmental sensors under METI’s 2025 IoT initiative. India and Southeast Asia are also adopting AuNP-enhanced sensors for food safety testing, guided by regulatory interest in quality monitoring. With its cost-effective production, population-driven healthcare demand, and aligned policy support, APAC is solidifying its position as the fastest-growing region.
China represents the fastest-growing market for gold nanoparticles globally, powered by aggressive government support through its 14th Five-Year Plan. Biotech firms in Shanghai, backed by the Chinese Academy of Sciences, are piloting rapid AuNP-based assays for infectious disease detection. Simultaneously, large-scale manufacturers explore AuNP applications in printed electronics, flexible displays, and conductive inks. Favourable trade policies and extensive government incentives further lower barriers to scaling up production. China’s integration of policy support, rapid manufacturing capabilities, and expanding healthcare demand ensures its continued dominance as a global production and application hub for AuNP technologies.
India’s gold nanoparticles industry is advancing rapidly, driven by targeted government programs. The Department of Biotechnology (DBT) and Department of Science and Technology (DST) initiated calls in late 2024 to establish GMP-grade green synthesis facilities in Bangalore and Pune. A breakthrough from Osmania University researchers in Hyderabad resulted in a patented AuNP-based rapid sepsis test capable of delivering results in just 30 minutes, supported by government funding. India’s growing academic excellence, government-backed translational research, and rising demand for affordable diagnostic solutions create a self-sustaining ecosystem. With expanding clinical infrastructure and lower-cost production capabilities, India is emerging as a key regional player in the global AuNP market.
Europe Market Trends
Europe remains a significant player in the global gold nanoparticles market, underpinned by advanced healthcare systems, strong regulatory policies, and industrial innovation hubs across Germany, the U.K., and France. The region’s steady growth is supported by both biomedical and industrial applications and substantial public funding. Initiatives like Horizon Europe’s 2025 NanoHealth program invested €200 million to advance AuNP applications in diagnostics and regenerative medicine.
- In electronics, companies like Bosch and Philips are piloting AuNP inks for flexible biosensors under EU-supported manufacturing programs. Europe’s well-coordinated regulatory frameworks, generous public funding, and industry-academia collaboration have positioned it as a key market for sustainable, high-value AuNP applications, particularly in precision diagnostics and next-gen healthcare.
The U.K. continues to advance its AuNP market leadership through strong public funding, clinical trials, and academic research. Programs such as Horizon Europe and Innovate UK provide continuous financial backing for nanoparticle-based healthcare innovations. NHS-backed trials actively investigate AuNP applications in rapid diagnostics, such as sepsis detection and infectious disease screening. Companies like BBI Solutions have integrated AuNPs into cutting-edge point-of-care testing kits, demonstrating commercial scalability. The U.K.’s world-class clinical infrastructure, proactive regulatory agencies, and highly integrated research networks ensure that it remains at the forefront of AuNP-enabled healthcare technologies, supporting sustained market growth.
Germany is a key innovation hub for biomedical and industrial AuNP applications. In partnership with Merck KGaA, the Fraunhofer Society launched a significant project in 2024 focused on commercialising AuNP-based contrast agents for photoacoustic imaging. Germany’s High-Tech Strategy includes substantial grants for advancing nanomedicine, green manufacturing processes, and GMP-grade AuNP production. Major industrial players like Tanaka Kikinzoku are scaling up production of water-soluble and imaging-grade nanoparticles. Germany’s robust regulatory standards, skilled workforce, and leadership in industrial biotech position it as a European innovation powerhouse, balancing clinical advancement with manufacturing excellence in the global AuNP market.
Type Insights
Water-soluble gold nanoparticles dominate the market, accounting for over 40% share, largely due to their compatibility with biomedical applications. Their ability to disperse effectively in aqueous solutions makes them highly suitable for drug delivery, bio-imaging, and in vitro diagnostics (IVD). Several diagnostic companies are integrating AuNP-based colourimetric sensors for early-stage cancer detection, demonstrating commercial viability. Advances in surface functionalisation enable these nanoparticles to be conjugated with targeting ligands like antibodies, enhancing specificity while minimising toxicity.
Such biocompatibility makes water-soluble AuNPs ideal carriers for chemotherapeutics like paclitaxel and doxorubicin and emerging photothermal therapies. Increased clinical trial activity and regulatory progress, particularly for AuNP-enhanced imaging modalities (CT, PET, photoacoustic imaging), further support their market leadership. As personalised medicine expands, water-soluble AuNPs remain central to healthcare innovation.
Application Insights
The imaging segment led the market in 2024 with a 21% revenue share and is the fastest-growing application, driven by gold nanoparticles' unique plasmonic and optical properties. Their localised surface plasmon resonance (LSPR) enables advanced imaging techniques such as photoacoustic imaging, OCT, and SERS. In 2024, innovations in gold nanocages allowed NIR-tunable imaging, significantly improving depth and diagnostic precision. Increasing clinical trials focus on AuNP-enhanced CT and MRI contrast agents.
Major companies like Sigma-Aldrich and Nanocomposix launched clinical-grade nanoparticle formulations in 2024, addressing rising demand from research institutions and hospitals. The growing prevalence of oncology and cardiovascular diseases drives strong adoption, supported by regulatory agencies accelerating non-invasive diagnostic approvals. This convergence of medical need, technological advancement, and favourable regulation ensures the imaging segment will continue robust double-digit growth into 2025 and beyond.
End-User Insights
The healthcare and pharmaceutical sector leads end-user demand for gold nanoparticles, driven by rising investments in nanomedicine, diagnostics, and precision drug delivery systems. AuNPs’ biocompatibility, ease of functionalisation, and optical properties make them ideal for targeted therapies, particularly in oncology, cardiovascular diseases, and infectious disease management. Pharmaceutical giants like GSK and Roche are actively collaborating with startups to develop AuNP-based immunoassays and drug delivery systems. Clinical trials using AuNPs as radiosensitizers in proton therapy and as carriers for chemotherapeutic agents continue to expand globally.
Additionally, increased funding for nanoparticle-enhanced point-of-care diagnostics reflects growing market confidence. As healthcare systems prioritise personalised and minimally invasive therapies, the demand for gold nanoparticles in the pharmaceutical sector is poised to maintain steady, high-growth momentum.
List of Key and Emerging Players in Gold Nanoparticles Market
- Nanopartz
- Nanocs
- nanoComposix
- BBI Solutions
- Cline Scientific
- Cytodiagnostics
- SigmaAldrich (Merck)
- Tanaka Technologies
- Goldsol
- Expedeon
- Metalor Technologies SA
- Solaris Nanosciences
- NanoHybrids
- American Elements
- SAT Nano
to learn more about this report Download Market Share
Recent Developments
- May 2025- Osmania University (India) developed and patented a rapid, low-cost sepsis diagnostic test using gold nanoparticles. The colourimetric assay delivers results in 30 minutes, backed by Department of Biotechnology funding.
- January 2025- National University of Singapore researchers introduced DNA-tagged, shape-specific AuNPs for precision cancer therapy and photothermal treatment, reported in ACS Nano, demonstrating enhanced tumour targeting accuracy
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 5.8 Billion |
| Market Size in 2025 | USD 6.4 Billion |
| Market Size in 2033 | USD 13.6 Billion |
| CAGR | 10.1% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Application, By End-User Industry |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Gold Nanoparticles Market Segments
By Type
- Water-soluble AuNPs
- Oil-soluble AuNPs
- Dual-phase (water + oil) soluble AuNPs
By Application
- Imaging
- Targeted drug delivery
- Sensors
- Invitro diagnostics
- Probes
- Catalysis
- Others
By End-User Industry
- Healthcare & Pharmaceuticals
- Electronics & Electrical
- Chemicals
- Others (Cosmetics, Personal Care, Glass, Photometry)
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Anantika Sharma
Research Practice Lead
Anantika Sharma is a research practice lead with 7+ years of experience in the food & beverage and consumer products sectors. She specializes in analyzing market trends, consumer behavior, and product innovation strategies. Anantika's leadership in research ensures actionable insights that enable brands to thrive in competitive markets. Her expertise bridges data analytics with strategic foresight, empowering stakeholders to make informed, growth-oriented decisions.










































