Healthcare Third-Party Logistics (3PL) Market Size, Share & Trends Analysis Report By Product (Biopharmaceuticals, Pharmaceuticals, Medical Device), By Service (Transportation, Warehousing & Storage, Others), By Supply Chain (Cold Chain, Non-Cold Chain Logistics) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Healthcare Third-Party Logistics (3PL) Market Overview
The global healthcare third-party logistics (3PL) market size is estimated at USD 278.79 billion in 2025 and is projected to reach USD 542.53 billion by 2034, growing at a CAGR of 7.72% during the forecast period. Strong growth of the market is propelled by the increasing globalisation of pharmaceutical supply chains, rising demand for temperature-sensitive biopharmaceuticals and vaccines, and growing outsourcing of logistics operations by healthcare manufacturers to enhance operational efficiency and focus on core competencies.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 41.37% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 9.26%.
- Based on Product, the biopharmaceuticals segment dominated the market in 2025 with a revenue share of 51.27%.
- Based on Service, the warehousing & storage dominated the market in 2025, with a revenue share of 48.16%.
- Based on the Supply Chain, the cold chain segment is anticipated to register the fastest CAGR of 8.82%.
- The U.S. dominates the global healthcare third-party logistics (3PL) market, valued at USD 99,663.20 million in 2024 and reaching USD 1,06,928.65 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
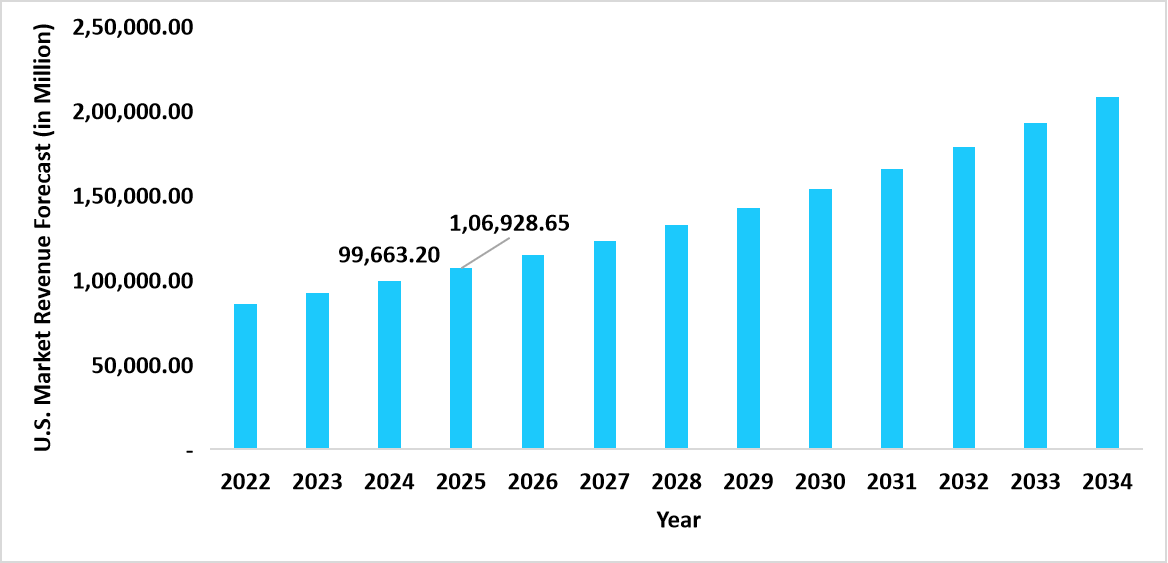
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 278.79 billion
- 2034 Projected Market Size: USD 542.53 billion
- CAGR (2025 to 2034): 7.72%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The healthcare third-party logistics (3PL) market refers to the outsourcing of logistics and supply chain operations by healthcare manufacturers, distributors, and suppliers to specialised service providers to ensure the efficient, compliant, and secure movement, storage, and distribution of healthcare products. The market, segmented by product, includes biopharmaceuticals, pharmaceuticals, comprising vaccines, biosimilars, plasma-derived products, and others and medical devices, categorised into Class I, II, and III. By service, it covers transportation (air freight, sea freight, and overland), warehousing & storage, and other value-added logistics functions such as packaging, labelling, and order fulfilment. By supply chain, it is divided into cold chain and non-cold chain logistics, supporting both temperature-sensitive products like biopharmaceuticals and vaccines, and general healthcare goods such as medical supplies and OTC products. Overall, healthcare 3PL providers play a critical role in enhancing supply chain efficiency, maintaining product integrity, ensuring regulatory compliance, and reducing operational costs across the global healthcare ecosystem.
Latest Market Trends
Adoption of Cold Chain Automation and IoT-Enabled Monitoring Systems
A major trend in the healthcare third-party logistics (3PL) market is the increasing adoption of automation and Internet of Things (IoT) technologies to enhance cold chain efficiency and reliability. Logistics providers are deploying automated storage and retrieval systems (ASRS), GPS tracking, and IoT-enabled temperature sensors to ensure real-time visibility and precise control over biologics, vaccines, and other temperature-sensitive products. For instance, several leading 3PL providers implemented IoT-driven monitoring systems that alert operators to any deviation in temperature or humidity levels, reducing spoilage and ensuring compliance with regulatory standards. This indicates that the integration of automation and IoT technologies has transformed healthcare logistics by minimising human error, improving traceability, and ensuring the integrity of high-value pharmaceutical products throughout the supply chain.
Integration of Sustainability and Green Logistics Practices
The key trend in the healthcare 3PL market is the growing integration of sustainability-driven logistics practices. Logistics companies are increasingly adopting eco-friendly measures such as electric delivery vehicles, biodegradable packaging, and energy-efficient warehouses to reduce carbon emissions. Several global healthcare logistics providers introduced route optimisation and load consolidation software to minimise fuel consumption while maintaining timely pharmaceutical delivery. This demonstrated that the transition toward green logistics is reshaping the competitive landscape by enabling 3PL providers to align with environmental regulations, enhance corporate responsibility, and appeal to pharmaceutical clients seeking sustainable and compliant logistics partners.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 278.79 Billion |
| Estimated 2026 Value | USD 299.31 Billion |
| Projected 2034 Value | USD 542.53 Billion |
| CAGR (2026-2034) | 7.72% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Agility Logistics, Barrett Distribution, Cardinal Health, Cencora, CEVA Logistics |
to learn more about this report Download Free Sample Report
Market Driver
Rising Demand for Temperature-Sensitive Biopharmaceuticals
A key driving factor in the healthcare 3PL market is the increasing global demand for temperature-sensitive biopharmaceuticals, including vaccines, biologics, and gene therapies. These products require precise temperature control and validated handling processes to maintain their safety and efficacy throughout distribution. For instance, leading pharmaceutical manufacturers partnered with specialized 3PL providers equipped with ultra-cold storage systems, insulated packaging, and real-time monitoring to ensure end-to-
end cold chain integrity. Such developments highlighted that the growing dependence on advanced cold chain logistics is driving the expansion of the healthcare 3PL market by enhancing delivery efficiency, ensuring regulatory compliance, and supporting the large-scale distribution of critical life-saving therapies.
Market Restraint
Stringent Regulatory Requirements and High Compliance Costs
A major restraint in the healthcare 3PL market is the stringent regulatory framework governing the storage and transportation of pharmaceuticals and biologics. Logistics providers must adhere to international standards such as Good Distribution Practices (GDP) and Good Manufacturing Practices (GMP), which demand extensive documentation, audits, and temperature validation. For example, global providers complied with region-specific regulations that vary across the U.S., Europe, and Asia, adding layers of operational complexity and cost. This reflected that high compliance costs and the demand for continuous certification acted as barriers for small and mid-sized logistics providers, limiting their scalability and profitability in the global healthcare logistics sector.
Market Opportunity
Expansion of Healthcare Logistics in Emerging Markets
An opportunity in the healthcare 3PL market lies in the expansion of logistics operations across emerging economies such as India, China, Brazil, and Southeast Asia. These regions witnessed rapid growth in pharmaceutical manufacturing, rising healthcare spending, and government investments in medical infrastructure. For instance, several global logistics firms entered into partnerships with local distributors and established regional cold chain hubs to support the growing demand for efficient pharmaceutical distribution. This suggested that strategic expansion into emerging markets enables 3PL providers to tap into high-growth regions, strengthen global reach, and offer technologically advanced logistics solutions tailored to local regulatory and infrastructure demands, thereby driving long-term market growth.
Regional Analysis
The North America region dominated the market with a revenue share of 41.37% in 2025, driven by advanced logistics infrastructure, strong regulatory compliance, and extensive outsourcing of distribution services by pharmaceutical companies. The region’s leadership is further supported by the widespread integration of digital logistics technologies such as warehouse automation, real-time temperature monitoring, and AI-driven supply chain analytics, which enhance efficiency and traceability across pharmaceutical and biotechnology shipments. These advancements have enabled healthcare organizations to manage large-scale vaccine distribution, biologics transport, and precision medicine logistics with greater speed and safety, reinforcing North America’s dominance in the global market.
In the U.S., market growth is primarily driven by the increasing adoption of value-added logistics solutions, including reverse logistics, cold chain packaging, and compliance consulting services tailored for the healthcare industry. The U.S. Food and Drug Administration’s (FDA) emphasis on Good Distribution Practices (GDP) compliance has encouraged healthcare manufacturers to partner with certified 3PL providers that offer secure, validated, and temperature-controlled transportation systems. This trend has enabled pharmaceutical companies to streamline distribution, reduce wastage, and ensure patient safety, thereby expanding the market growth.
Asia Pacific Market Insights
The Asia Pacific region is the fastest-growing market for healthcare 3PL, projected to register a CAGR of 9.26% during the forecast period. The rapid expansion is attributed to increasing pharmaceutical production in countries such as China, India, and South Korea, coupled with the modernization of healthcare logistics infrastructure. Government investments in smart logistics systems, automation, and cold chain network expansion are accelerating market growth. Moreover, the surge in biologics, vaccines, and clinical trial shipments across the region has intensified the demand for specialized, compliant 3PL services that ensure quality and product integrity throughout the supply chain.
In India, the healthcare 3PL market is growing rapidly due to the expansion of domestic pharmaceutical manufacturing and the government’s focus on enhancing healthcare distribution networks. Initiatives such as the National Logistics Policy (NLP) and the development of multi-modal logistics parks have improved transportation efficiency and reduced transit times for temperature-sensitive drugs. Additionally, partnerships between logistics providers and pharmaceutical firms are enabling end-to-end distribution solutions, from storage and packaging to last-mile delivery, strengthening India’s position as a major healthcare logistics hub in the Asia Pacific region.
Pie Chart: Regional Market Share, 2025
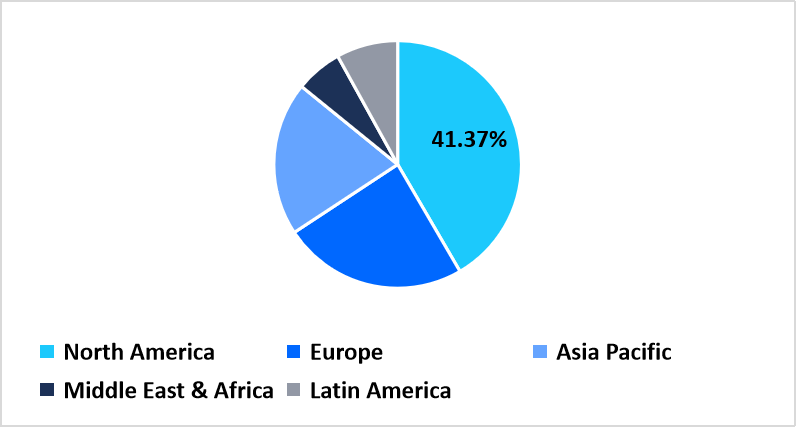
Source: Straits Research
Europe Market Insights
Europe holds a substantial share of the global healthcare 3PL market due to the region’s potent pharmaceutical manufacturing base and the rapid digital transformation of logistics networks. The implementation of the EU Falsified Medicines Directive (FMD) and the growing adoption of serialization and track and trace systems have accelerated the shift toward fully transparent and technology-integrated supply chains. Moreover, the rising focus on sustainable logistics practices such as green warehousing, carbon-neutral transport, and recyclable cold chain materials has further enhanced operational efficiency and compliance in pharmaceutical distribution across the continent.
In the United Kingdom, the healthcare 3PL market is being propelled by ongoing investments in digital logistics and the expansion of specialized distribution hubs. The increasing adoption of cloud-based logistics management systems and advanced automation tools has enabled more accurate inventory tracking and predictive demand forecasting. Additionally, the UK government’s focus on post-Brexit healthcare supply chain resilience has prompted logistics providers to establish regional facilities and improve cross-border efficiency, ensuring uninterrupted pharmaceutical flow and reinforcing the market’s stability and growth.
Middle East and Africa Market Insights
In the Middle East and Africa, market growth is primarily driven by increasing investment in cold chain infrastructure and the growing demand for temperature-controlled logistics solutions to support pharmaceutical imports and vaccine distribution. The expansion of healthcare systems, along with the region’s ongoing efforts to improve storage and distribution standards, has led to greater collaboration between governments and international logistics providers. Additionally, the introduction of digital tracking technologies and mobile-based supply chain monitoring platforms is enabling real-time visibility and improving the reliability of pharmaceutical delivery, even in remote and resource-limited areas.
In the UAE, the healthcare 3PL market is witnessing rapid development due to strategic government initiatives aimed at strengthening the nation’s role as a regional healthcare distribution hub. The Dubai Industrial Strategy and the establishment of logistics free zones dedicated to pharmaceutical warehousing have attracted leading global 3PL providers. Moreover, the integration of smart logistics solutions and AI-based warehouse management systems is supporting seamless operations for biologics and medical devices, positioning the UAE as a key logistics gateway for the broader Middle East and African markets.
Latin America Market Insights
In Latin America, the healthcare 3PL market is expanding steadily, supported by the localization of pharmaceutical distribution and the modernization of logistics infrastructure. Governments across Brazil, Mexico, and Argentina are encouraging private sector investment in healthcare warehousing, packaging, and cold chain transport to strengthen medicine availability and reduce supply delays. The growing adoption of digital logistics platforms for shipment tracking and inventory management has further enhanced operational transparency and regulatory compliance in the region.
In Brazil, the healthcare 3PL market is being driven by government initiatives promoting domestic pharmaceutical logistics and public-private partnerships aimed at improving supply chain efficiency. The National Health Surveillance Agency (ANVISA) has enforced stricter guidelines on drug traceability, prompting logistics providers to adopt serialization and electronic tracking systems. Furthermore, Brazil’s focus on expanding regional distribution centers has improved the accessibility of essential medicines across remote regions, thereby strengthening its role as a central logistics hub in Latin America’s healthcare ecosystem.
Product Insights
The biopharmaceuticals segment dominated the market in 2025, with a 51.27% share, owing to the rising global demand for biologics, vaccines, and advanced therapies that require specialized handling, storage, and regulatory compliance. The increasing prevalence of chronic diseases and the expansion of pharmaceutical R&D activities have further fueled the demand for efficient biopharma logistics solutions.
The medical device segment is anticipated to register the fastest CAGR of 8.26%, owing to the growing distribution of complex and high-value medical equipment, advancements in medical technology, and the rising adoption of e-commerce and direct-to-hospital delivery models. Additionally, the increasing outsourcing of supply chain operations by device manufacturers to ensure cost efficiency and compliance with stringent safety regulations supports this segment’s growth.
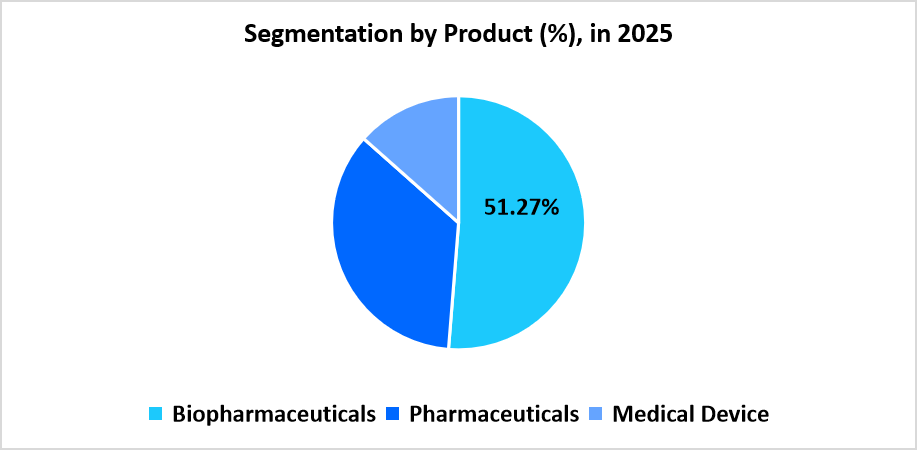
Source: Straits Research
Service Insights
The warehousing & storage segment dominated the market in 2025, with 48.16% share, due to the expansion of storage infrastructure equipped with advanced inventory management systems, temperature monitoring, and regulatory-compliant facilities for biopharmaceuticals and medical devices. The demand for safe, traceable, and efficient storage solutions has been amplified by rising product complexity and global distribution networks.
The transportation segment is anticipated to register the fastest CAGR of 9.07% during the forecast period, driven by the increasing demand for time-sensitive deliveries, growing adoption of digital freight platforms, and the integration of IoT and telematics for route optimization and real-time shipment tracking. Furthermore, the shift toward sustainable logistics through electric and hybrid vehicle adoption is accelerating segment expansion.
Supply Chain Insights
The non-cold chain logistics segment dominated the market growth due to the extensive demand for the transportation and distribution of pharmaceutical products, medical devices, and consumables that do not require temperature-controlled environments. This segment benefits from the growing volume of generic drugs, over-the-counter (OTC) products, and medical supplies, along with the increasing outsourcing of logistics services by healthcare manufacturers to enhance efficiency and reduce operational costs.
The cold chain segment is anticipated to register the fastest CAGR of 8.82%, owing to the increasing demand for temperature-controlled logistics solutions that ensure the safe storage and transportation of biopharmaceuticals, vaccines, and other temperature-sensitive healthcare products.
Competitive Landscape
The global healthcare third-party logistics (3PL) market is fragmented, with a combination of established logistics giants and emerging specialized providers driving competition. Major players such as DHL Supply Chain, UPS Healthcare, FedEx Supply Chain, Kuehne + Nagel, DB Schenker, and others dominate through global distribution networks, advanced cold-chain infrastructure, and regulatory compliance expertise. Additionally, emerging companies such as Kinesis Medical, Agility Logistics, and Kerry Logistics are gaining traction by offering innovative temperature-controlled solutions, clinical trial logistics, and region-specific services, strengthening their presence in the healthcare 3PL market.
Kinesis Medical B.V.: An emerging market player
Kinesis Medical, a specialized healthcare logistics provider, is emerging as a key player in the Healthcare Third-Party Logistics market with a focus on temperature-sensitive and clinical trial logistics. The company leverages advanced cold-chain technologies and real-time tracking systems to ensure the safe and compliant transportation of pharmaceuticals and biologics.
- In 2024, Kinesis Medical, expanded its European operations by opening a state-of-the-art temperature-controlled distribution center, enhancing its capacity to serve clinical trials and specialized pharmaceutical shipments. Through innovation and regional expansion, Kinesis Medical is establishing itself as a prominent emerging player in the healthcare 3PL market.
List of Key and Emerging Players in Healthcare Third-Party Logistics (3PL) Market
- Agility Logistics
- Barrett Distribution
- Cardinal Health
- Cencora
- CEVA Logistics
- DB Schenker
- DHL Group
- FedEx
- Freight Logistics Solutions
- Hellmann Worldwide Logistics
- Kerry Logistics Network Ltd.
- Kinesis Medical B.V.
- KUEHNE + NAGEL
- Nippon Express Co., Ltd.
- Ryder System, Inc.
- SF Express
- Sinotrans Limited
- United Parcel Service of America, Inc.
- XPO Logistics, Inc.
- Others
Strategic Initiatives
- September 2025: DHL Supply Chain agreed to acquire SDS Rx, a provider of final-mile delivery and specialized healthcare transportation for long-term care and specialty pharmacies, radiopharmacies, and health system networks to strengthen its life sciences and healthcare (LSHC) capabilities.
- April 2025: EVERSANA, a leading healthcare service provider, expanded its pharmaceutical third-party logistics (3PL) operations to meet growing client demands through the opening of a new 358,000-square-foot, cGDP-certified distribution center.
- April 2025: DHL Group invested USD 2.3 billion over the next five years to enhance its logistics capabilities in the life sciences and healthcare sector.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 278.79 Billion |
| Market Size in 2026 | USD 299.31 Billion |
| Market Size in 2034 | USD 542.53 Billion |
| CAGR | 7.72% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Service, By Supply Chain |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Healthcare Third-Party Logistics (3PL) Market Segments
By Product
- Biopharmaceuticals
-
Pharmaceuticals
- Vaccines
- Biosimilars
- Plasma Derived Products
- Others
-
Medical Device
- Class I
- Class II
- Class III
By Service
-
Transportation
- Air Freight
- Sea Freight
- Overland
- Warehousing & Storage
- Others
By Supply Chain
- Cold Chain
- Non-Cold Chain Logistics
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































