HPV Associated Disorders Market Size, Share & Trends Analysis Report By Type (Cervical Cancer, Cervical Intraepithelial Neoplasia, Anal Intraepithelial Neoplasia, Anal Cancer, Genital Warts, Others), By Treatment Type (Vaccines, Anti-viral Drugs, Others), By Distribution Channel (Hospital Pharmacies, Drug Stores & Retail Pharmacies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
HPV Associated Disorders Market Overview
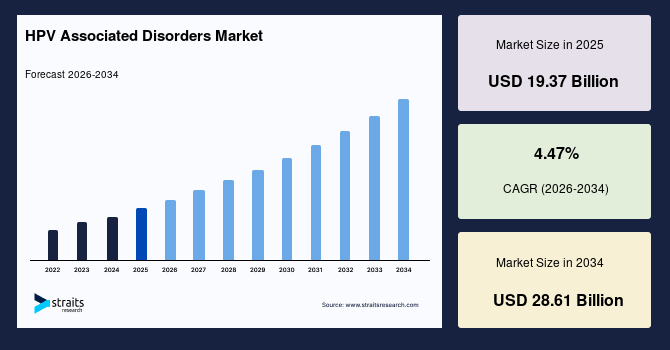
The global HPV associated disorders market size is estimated at USD 19.37 billion in 2025 and is projected to reach USD 28.61 billion by 2034, growing at a CAGR of 4.47% during the forecast period. The remarkable market growth is attributed to the expanding pipeline of targeted immunotherapies and therapeutic vaccines designed to treat persistent high-risk HPV infections.
Key Market Trends & Insights
- North dominated the global market, accounting for a 37.56% share in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 5.93%.
- Based on type, the cervical cancer segment dominated the market in 2025, accounting for a revenue share of 34.67%.
- By treatment type, the anti-viral drugs segment is estimated to grow at the fastest pace with a CAGR of 5.08% in 2026-2034.
- By distribution channel, the hospital pharmacies segment dominated the market, accounting for 49.16% revenue share in 2025.
- The U.S. dominates the market, valued at USD 6.30 billion in 2024 and reaching USD 6.55 billion in 2025.
Table: U.S. HPV Associated Disorders Market Size (USD Million)
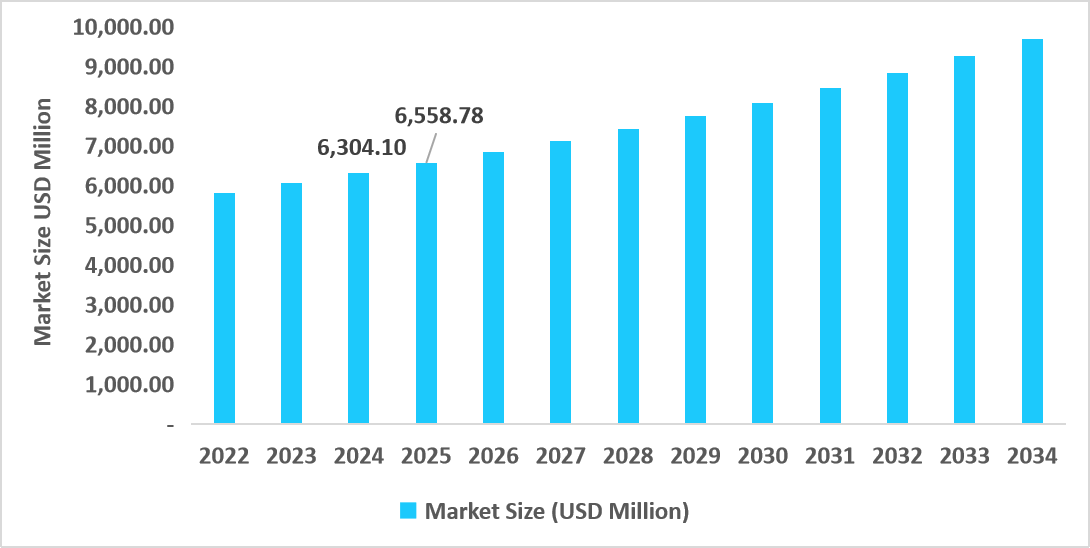
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 19.37 billion
- 2034 Projected Market Size: USD 28.61 billion
- CAGR (2026-2034): 4.47%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The HPV associated disorders market comprises a wide spectrum of conditions caused by human papillomavirus, including cervical cancer, cervical and anal intraepithelial neoplasia, anal cancer, genital warts, and related disorders. The market encompasses preventive and therapeutic solutions such as vaccines, antiviral drugs, and other supportive treatments. These products are distributed through hospital pharmacies, drug stores, retail pharmacies, and additional healthcare channels to support the prevention and management of HPV related diseases.
Latest Market Trends
Growing Shift toward Therapeutic HPV Vaccines for Persistent Infections
A key recent trend in the HPV associated disorders market is the accelerating shift from solely preventive vaccination to the development of therapeutic HPV vaccines aimed at treating persistent high-risk HPV infections and early-stage lesions. Various late-stage clinical candidates, including DNA and vector-based immunotherapies, have shown promising immune-boosting responses. This trend reflects rising demand for targeted treatment options that address unmet demand beyond traditional prevention.
Increasing Advancement and Adoption of Novel Multivalent HPV Vaccines
Another major trend in the HPV associated disorders market is the rising development and regulatory progress of next-generation multivalent HPV vaccines designed to offer broader protection against additional HPV strains. These advanced vaccines aim to improve immunity, enhance population coverage, and further reduce HPV related cancer incidence. Ongoing clinical evaluations and strong global interest highlight growing emphasis on expanding preventive capabilities and strengthening vaccination strategies to address evolving HPV epidemiology.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 19.37 Billion |
| Estimated 2026 Value | USD 20.16 Billion |
| Projected 2034 Value | USD 28.61 Billion |
| CAGR (2026-2034) | 4.47% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Merck & Co., Inc., GSK plc, HoffmannâLa Roche Ltd., Pfizer Inc., AstraZeneca |
to learn more about this report Download Free Sample Report
Market Drivers
Rising global Burden of HPV-related cancers
The increasing global burden of HPV related cancers, such as cervical and oropharyngeal cancers, has intensified the demand for preventive and therapeutic interventions. Growing detection of high-risk HPV strains and rising screening coverage have highlighted the urgency for effective vaccines, antiviral therapies, and diagnostic solutions. As healthcare systems prioritize cancer prevention and early treatment, the heightened disease prevalence accelerates market growth.
Market Restraints
Limited Access to HPV Vaccination in Low-Resource Regions
A major restraint in the HPV associated disorders market is the limited accessibility of HPV vaccines in low and middle-income countries, where supply gaps and affordability issues restrict coverage. Despite global vaccination initiatives, millions remain unvaccinated due to inconsistent procurement and distribution. A recent instance occurred in 2024 when several African nations reported delays in receiving HPV vaccine shipments, leading to postponed immunization campaigns and reduced protection for adolescent girls, thereby hindering market expansion.
Market Opportunity
Expansion of Single-Dose HPV Vaccination Programs
The rapid adoption of single-dose HPV vaccination schedules, which enhances affordability and simplifies large-scale immunization, presents scope for market growth. In 2024, the World Health Organization approved a new single-dose HPV vaccine, allowing countries to expand coverage more efficiently. This advancement reduces logistical challenges, supports wider vaccination uptake in underserved regions, and strengthens global prevention efforts, creating substantial growth potential for the market.
Regional Analysis
North America dominated the market in 2025, accounting for 37.56% market share, owing to the widespread adoption of integrated electronic health record (EHR) systems that facilitate coordinated HPV vaccination reminders, screening follow-ups, and patient tracking. This digital integration enhances preventive care efficiency, increases vaccination rates, and strengthens regional market growth.
The U.S. HPV associated disorders market is supported by the implementation of school and workplace based HPV vaccination initiatives, which provide convenient access for adolescents and young adults. These programs improve immunization coverage, enhance early prevention efforts, and contribute to the expansion of the U.S. market.
Asia Pacific Market Insights
Asia Pacific is emerging as a fastest-growing region with a CAGR of 5.93% from 2026-2034. The region benefits from rising government-supported mass immunization programs targeting adolescent girls in rural and semi-urban areas, which expand vaccine accessibility, increase awareness, and accelerate adoption, positioning the region as the fastest-growing market for HPV prevention and treatment solutions.
China HPV HPV-associated disorders market is expanding rapidly, driven by the recent approval and local production of domestically manufactured HPV vaccines, which reduces costs and supply dependency on imports. This enhances vaccine accessibility, supports large-scale national immunization programs, and notably boosts market adoption across urban and rural populations.
Regional Market share (%) in 2025
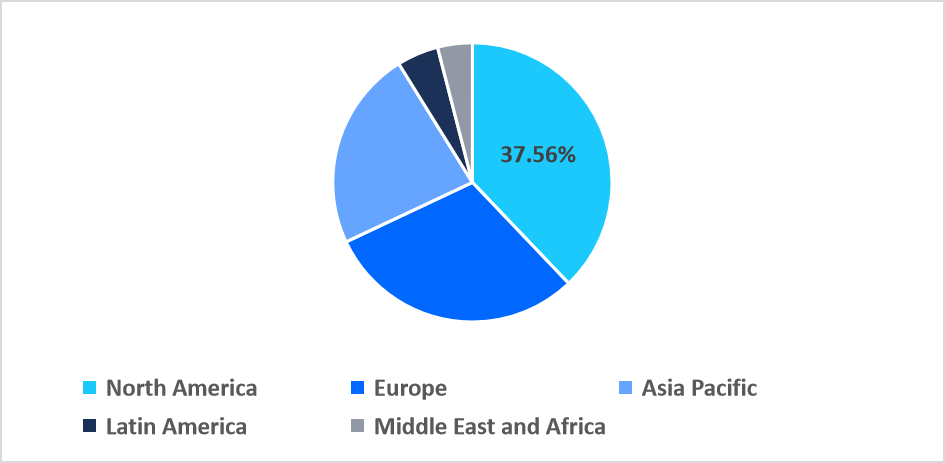
Source: Straits Research
Europe Market Insights
Europe market growth is driven by the integration of advanced HPV DNA screening into national cervical cancer programs, enabling earlier detection of high risk infections. This proactive screening approach increases demand for follow-up diagnostics, vaccines, and therapeutic interventions, supporting sustained market growth across European healthcare systems.
A key factor driving growth in the Germany HPV associated disorders market is the success of the national immunization program, which reduced HPV related conditions. Following its implementation, high-grade cervical lesions, anogenital warts, and vaginal precancer cases declined markedly, strengthening demand for continued vaccination and preventive healthcare initiatives.
Latin America Market Insights
Latin America market growth is supported by the region’s high acceptance of HPV vaccines. In 2024, studies reported vaccination acceptance rates around 84%, well above global averages, boosting immunization coverage and supporting market expansion for preventive and therapeutic HPV solutions.
In Argentina, the HPV associated disorders market is strengthened by the increased HPV prevalence among adolescents. Studies indicate over 56% of unvaccinated 15‑ to 17‑year-old girls carry HPV, including 42% with risk strains, which, in turn, increases demand for vaccination, screening, and early treatment interventions.
Middle East and Africa Market Insights
Middle East and Africa are witnessing steady market expansion, supported by the implementation of single dose HPV vaccination campaigns. These programs, supported by WHO, have improved vaccine accessibility, reduced logistical challenges, and accelerated coverage, particularly in underserved populations, enhancing market expansion.
In Saudi Arabia, the HPV associated disorders market is driven by the high prevalence of HPV types, including HPV‑16, HPV‑18, and HPV‑35, among women. This elevated infection rate increases demand for vaccination, screening, and therapeutic interventions, boosting market expansion.
Type Insights
The cervical cancer segment dominated the market and accounted for a 33.04% revenue share in 2025. This growth is driven by increasing adoption of AI-enabled colposcopy platforms that enhance early lesion detection in low-resource settings. These systems improve diagnostic accuracy, expand screening capacity, and accelerate clinical decision-making, strengthening demand for targeted cervical cancer treatments.
The cervical intraepithelial neoplasia segment is anticipated to witness the fastest growth, registering a CAGR of 9.04% during the forecast period. This growth is supported by the rising detection of low and intermediate-grade CIN cases due to expanded HPV screening coverage among younger women, especially those undergoing routine follow-up after vaccination. This trend increases CIN case identification earlier in the disease pathway, boosting demand for CIN-specific treatment services.
By Type Market Share (%), 2025
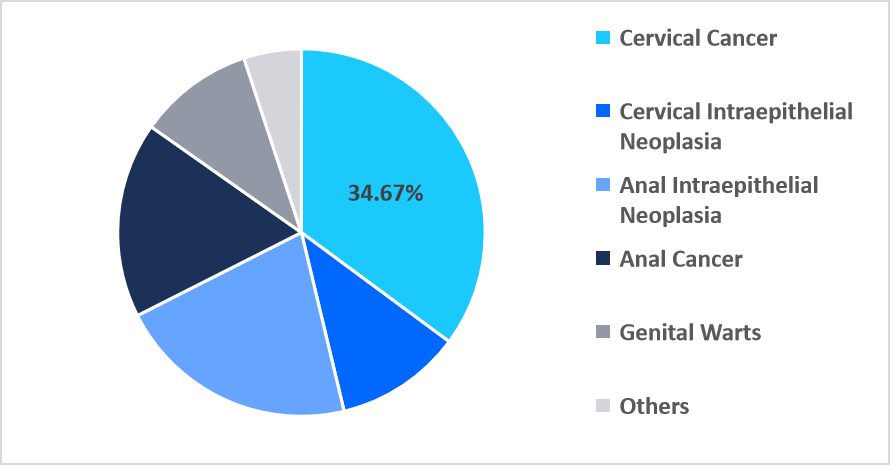
Source: Straits Research
Treatment Type Insights
The vaccines segment accounted for the largest market share of 64.05% in 2025. This dominance is attributed to the implementation of school-based vaccination mandates, which require both boys and girls to receive HPV immunization. This policy shift increases vaccination volume, broadens population coverage, and strengthens long-term demand for HPV vaccines.
The anti-viral drugs segment is projected to register the fastest CAGR growth of 5.08% during 2026-2034, owing to the development of broad-spectrum oral antivirals targeting multiple high-risk HPV strains, enabling outpatient management of persistent infections and early lesions, which increases treatment adoption and accelerates market expansion in both developed and emerging regions.
Distribution Channel Insights
The hospital pharmacies segment dominated the market in 2025 with a revenue share of 49.16% This growth is attributed to the increasing integration of onsite HPV vaccination and screening programs within hospital networks, allowing simultaneous diagnosis, treatment, and preventive care, which enhances convenience, patient adherence, and overall demand through hospital-based distribution channels.
The drug stores & retail pharmacies segment is expected to witness the fastest CAGR growth during the forecast timeframe, due to rising availability of over-the-counter medications, enabling convenient, community-based access to early treatment, which expands market reach beyond traditional clinical settings.
Competitive Landscape
The global HPV HPV-associated disorders market is moderately fragmented, with leading pharmaceutical and biotechnology companies capturing notable market share. Key players, including Merck & Co., GlaxoSmithKline, Pfizer, Roche, and others, strengthen their positions through new vaccine approvals, strategic collaborations, R&D investments, and awareness programs. Focused efforts on expanding vaccination coverage, enhancing diagnostics, and developing therapeutic interventions further drive their competitive growth globally.
Inovio Pharmaceuticals, Inc.: An emerging market player
Inovio Pharmaceuticals, a U.S.-based biotechnology company, is emerging in the market with a focus on therapeutic HPV vaccines for persistent high-risk infections and precancerous lesions. In 2024, its VGX-3100 vaccine showed promising Phase 3 trial results in cervical intraepithelial neoplasia. By 2025, Inovio expanded global clinical trials, reflecting regulatory confidence and positioning the company as an innovative player in HPV treatment.
List of Key and Emerging Players in HPV Associated Disorders Market
- Merck & Co., Inc.
- GSK plc
- Hoffmann‑La Roche Ltd.
- Pfizer Inc.
- AstraZeneca
- Biocon Ltd.
- Lilly
- Sanofi
- Novartis AG
- Bristol Myers Squibb Company
- AbbVie Inc.
- Amgen Inc.
- Bavarian Nordic A/S
- Others
Strategic Initiative
- April 2025: Merck agreed with UNICEF to achieve its goal of vaccinating millions of girls against Human Papillomavirus (HPV) by 2025 with GAVI.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 19.37 Billion |
| Market Size in 2026 | USD 20.16 Billion |
| Market Size in 2034 | USD 28.61 Billion |
| CAGR | 4.47% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Treatment Type, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
HPV Associated Disorders Market Segments
By Type
- Cervical Cancer
- Cervical Intraepithelial Neoplasia
- Anal Intraepithelial Neoplasia
- Anal Cancer
- Genital Warts
- Others
By Treatment Type
- Vaccines
- Anti-viral Drugs
- Others
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































