Interleukin Inhibitors Market Size, Share & Trends Analysis Report By Type (IL-1 Inhibitors, IL-2 Inhibitors, IL-5 Inhibitors, IL-6 Inhibitors, IL-17 Inhibitors, IL-23 Inhibitors, Other Types), By Route of Administration (Subcutaneous (SC), Intravenous (IV)), By Application (Rheumatoid Arthritis, Psoriasis, Inflammatory Bowel Disease (IBD), Asthma, Other Applications), By End Use (Hospitals, Specialty Clinics, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Interleukin Inhibitors Market Overview
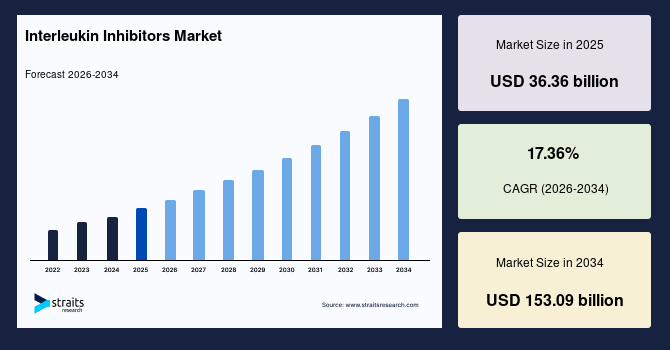
The global interleukin inhibitors market size is estimated at USD 36.36 billion in 2025 and is projected to reach USD 153.09 billion by 2034, growing at a CAGR of 17.36% during the forecast period. Remarkable growth of the market is driven by the rapid adoption of targeted biologics, expansion of approved indications across autoimmune and inflammatory diseases, and continuous advancement in cytokine-specific drug development.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 12% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 19.36%.
- By Type, the IL-23 inhibitors segment dominated the market with a revenue share of 28.35%.
- By Route of Administration, the subcutaneous segment dominated the market.
- By Application, the psoriasis segment dominated the market, with a revenue share of 40.12% share.
- By End Use, hospitals dominated the market in 2025 with a market share of 65.23%.
- The U.S. dominates the global interleukin inhibitors market, valued at USD 11.15 billion in 2024 and reaching USD 13.04 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
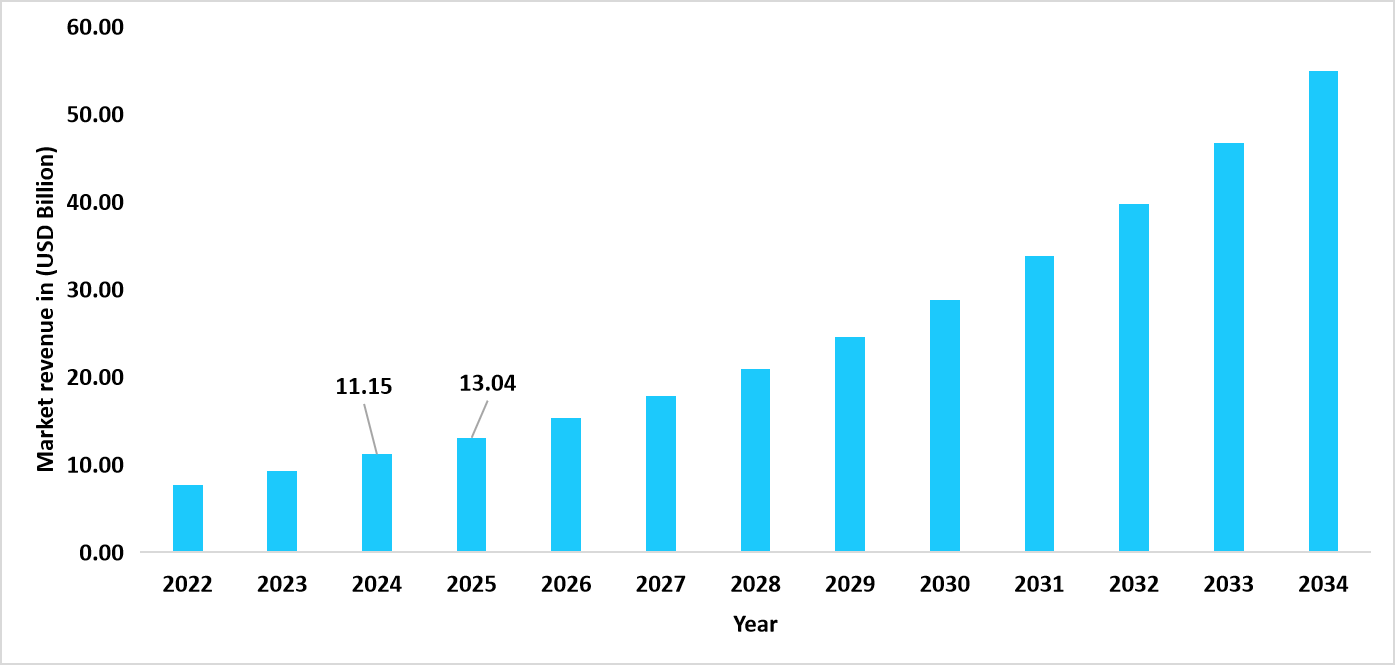
Source: Straits Research
Market Size & Forecast
- Market Size in 2025: 36.36 billion
- Market Size estimated in 2034: 153.09 billion
- CAGR: 17.36%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The interleukin inhibitors market comprises a class of targeted biologic therapies designed to block specific interleukin pathways involved in inflammatory and autoimmune disorders, offering precision treatment for chronic conditions with unmet clinical needs. The market spans multiple inhibitor types, including IL-1, IL-2, IL-5, IL-6, IL-17, IL-23, and other emerging molecules, each addressing distinct immune-mediated mechanisms. Products are delivered primarily through subcutaneous and intravenous routes, supporting diverse clinical requirements across acute and long-term care. These therapies are widely used for managing rheumatoid arthritis, psoriasis, inflammatory bowel disease, asthma and other immune-driven diseases, reflecting broad therapeutic utility. End-use demand is concentrated in hospitals and specialty clinics, with additional uptake across other healthcare settings as biologics become more accessible. Overall, the market is shaped by expanding biologic adoption, growing prevalence of autoimmune diseases, and ongoing innovation in cytokine-targeted drug development.
Latest Market Trends
Growing Integration of Combination Immunomodulation Strategies
A prominent trend shaping the interleukin inhibitors market landscape is the rising inclination toward combination therapy models that target multiple inflammatory pathways simultaneously. Clinicians are increasingly pairing interleukin inhibitors with JAK inhibitors, targeted synthetic agents, and advanced small-molecule immunotherapies to address complex autoimmune conditions that do not respond adequately to monotherapy. This integrated approach is gaining recognition for its ability to provide more durable disease suppression, reduce flare frequency, and broaden treatment effectiveness for patients exhibiting heterogeneous immune responses.
Increasing Adoption of Personalized Cytokine-based Treatment Selection
The market is witnessing growing implementation of biomarker-driven clinical decision-making, where patient-specific cytokine signatures and inflammatory markers guide the choice of interleukin-targeting biologics. Advances in molecular diagnostics and proteomic profiling are making it possible to map individual immune patterns more accurately. As a result, prescribers can tailor interleukin inhibitor regimens with greater precision, optimizing response rates and minimizing trial-and-error prescribing. This trend reflects a broader movement in immunology toward highly individualized treatment algorithms.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 36.36 billion |
| Estimated 2026 Value | USD 42.54billion |
| Projected 2034 Value | USD 153.09 billion |
| CAGR (2026-2034) | 17.36% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | AbbVie Inc., Johnson & Johnson, Novartis AG, Eli Lilly and Company, Amgen Inc. |
to learn more about this report Download Free Sample Report
Market Driver
Expansion of Clinical Research Into Rare and Under-treated Inflammatory Diseases
A key force driving market growth is the significant escalation in clinical research programs examining interleukin inhibition market across lesser-known or historically under-treated inflammatory disorders. Pharmaceutical developers are extending trials into areas such as systemic fibrotic syndromes, neuromuscular inflammatory disorders, and niche dermatologic conditions where unmet therapeutic needs are substantial. Broadening the investigational pipeline not only expands the eligible patient pool but also positions interleukin-targeting therapies as versatile solutions in previously overlooked segments of inflammatory care.
Market Restraint
Complexity and Cost Challenges Associated With Maintaining Cold-Chain Stability
One of the notable restraints for the market is the stringent requirement for uninterrupted temperature-controlled handling from production to final administration. Interleukin inhibitors are highly sensitive biologics that depend on robust cold-chain infrastructure to preserve safety and efficacy. Breakdowns in logistics, particularly across long shipping routes or multi-tier distribution channels, increase the risk of product degradation and elevate operational costs for manufacturers and distributors. These challenges create accessibility barriers, especially in markets with limited cold-chain capacity.
Market Opportunities
Advancements In Next-generation Self-Administration and High-concentration Delivery Systems
A compelling opportunity is emerging from innovation in biologic delivery technologies, particularly high-concentration formulations and wearable injector platforms designed for simplified patient self-administration. These systems aim to reduce dosing volumes, minimize injection discomfort, and lower the frequency of administration, which are the key attributes that improve convenience for individuals on long-term maintenance therapy. As these next-generation delivery solutions become more widely available, they are expected to significantly expand patient adherence and unlock new growth potential across outpatient treatment settings.
Regional Analysis
North America dominated the global interleukin inhibitors market landscape with a 40.12% share, driven by dense concentrations of biopharma headquarters, clinical trial sites, and advanced biologics manufacturing capacity. The region benefited from broad insurer reimbursement frameworks, phased launch strategies, and high physician awareness of targeted cytokine therapies, which supported rapid adoption across specialty clinics and academic medical centers.
The U S market expanded on the back of a large pipeline of late-stage assets, frequent regulatory interactions, and extensive post-approval real-world studies that guided prescribing in dermatology, pulmonology, and rheumatology.
Asia Pacific Market Insights
Asia Pacific experienced strong growth of 19.36% during the forecast period, as improving healthcare infrastructure, increasing awareness of biologic therapies, and expanding specialty care capacity supported broader adoption of IL inhibitors. Market growth was reinforced by rising diagnosis rates for psoriasis, rheumatoid arthritis, and inflammatory bowel disease alongside expanding reimbursement schemes in several markets. Local manufacturing partnerships and licensing agreements helped reduce cost barriers and enabled wider distribution across emerging and mature APAC markets.
India saw growing use of interleukin inhibitors in tertiary hospitals and specialty clinics, driven by better diagnostic pathways and increasing physician familiarity with biologics. Strategic collaborations between multinational drugmakers and local distributors improved patient access and supported patient assistance programs that gradually expanded treatment uptake beyond metropolitan centers.
Regional Market share (%) in 2025
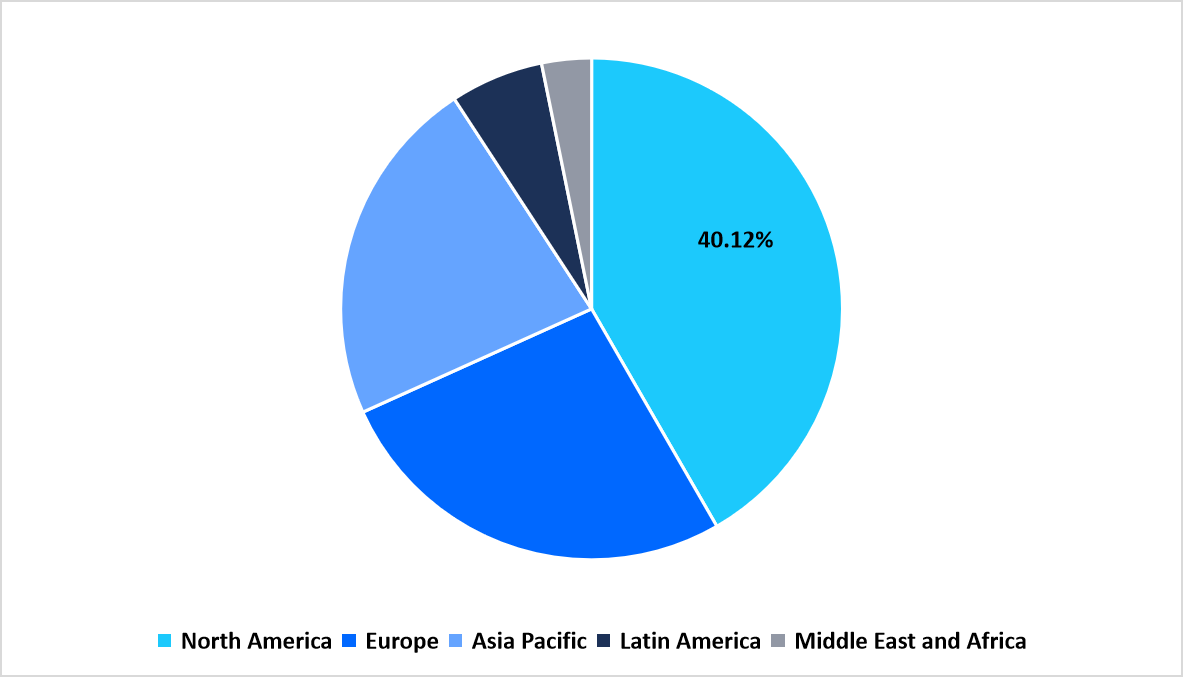
Source: Straits Research
Europe Market Insights
Europe’s market expansion was shaped by coordinated regulatory pathways, strong hospital-based specialist networks, and growing investments in precision medicine that guided targeted use of interleukin inhibitors. National health technology assessment processes and centralized procurement in several countries influenced formulary placements, while multi-center clinical collaborations and comparative effectiveness studies informed country-level adoption and guideline integration.
Germany exhibited steady adoption of IL inhibitors across dermatology and rheumatology settings, supported by comprehensive care networks and early incorporation of innovative therapies into regional treatment algorithms. Reimbursement negotiations and structured benefit assessments influenced which agents gained rapid penetration in outpatient specialty clinics.
Latin America Market Insights
Latin America recorded gradual but consistent market growth as increasing disease awareness, expansion of specialty clinics, and selective public and private reimbursement initiatives improved access to biologic therapies. Market expansion was often concentrated in urban centers where specialist physicians and diagnostic services were more accessible, and multinational companies focused on market entry strategies that included pricing arrangements and expanded distribution networks.
Brazil led regional uptake through growing private insurance coverage and established specialty centers that managed complex autoimmune conditions. Local regulatory approvals and targeted patient support programs enabled the expansion of interleukin inhibitor use in major metropolitan healthcare networks.
Middle East and Africa Market Insights
The Middle East and Africa market grew due to rising healthcare investment in specialty care, increased availability of advanced therapeutics in private hospitals, and government initiatives to modernize therapeutic formularies. Adoption varied across countries, with faster uptake in Gulf states and South Africa, where specialty infrastructure and reimbursement were more supportive.
South Africa showed measured adoption of interleukin inhibitors within private healthcare and referral centers for dermatology and rheumatology. Private payer frameworks and specialist prescribing patterns drove most of the market activity while public sector access remained more limited.
Type Insights
The IL-23 inhibitors segment dominated the market with a 28.35% share, supported by strong clinical acceptance across chronic inflammatory disorders and their sustained therapeutic outcomes.
The IL-17 inhibitors segment recorded the fastest CAGR of 17.12%, driven by their accelerating use in treatment-resistant cases and growing adoption across dermatology and rheumatology practices.
Route of Administration Insights
The subcutaneous (SC) route dominated the market with the largest share, reflecting its preference among patients and clinicians for convenient self-administered biologic therapy and reduced clinical dependency.
The intravenous (IV) route posted the fastest CAGR of 17.59%, supported by expanding administration of infusion-based biologics in controlled clinical environments for complex or severe immune-mediated conditions.
Application Insights
The psoriasis segment held the dominant position with a 40.12% share, reflecting extensive use of interleukin-targeting biologics among patients requiring long-term systemic therapy for moderate to severe disease.
The rheumatoid arthritis segment registered the fastest CAGR of 17.63%, underpinned by rising incorporation of interleukin inhibitors for patients inadequately responding to conventional DMARD therapies.
End Use Insights
The hospitals segment accounted for the largest share at 65.23%, supported by high treatment volumes, availability of multidisciplinary immunology teams, and expanded use of biologics in complex disease management.
The specialty clinics segment grew at the fastest CAGR of 7.98%, aided by the proliferation of focused dermatology, rheumatology, and gastroenterology centers offering biologic-driven therapeutic programs.
By End User Market Share (%), 2025
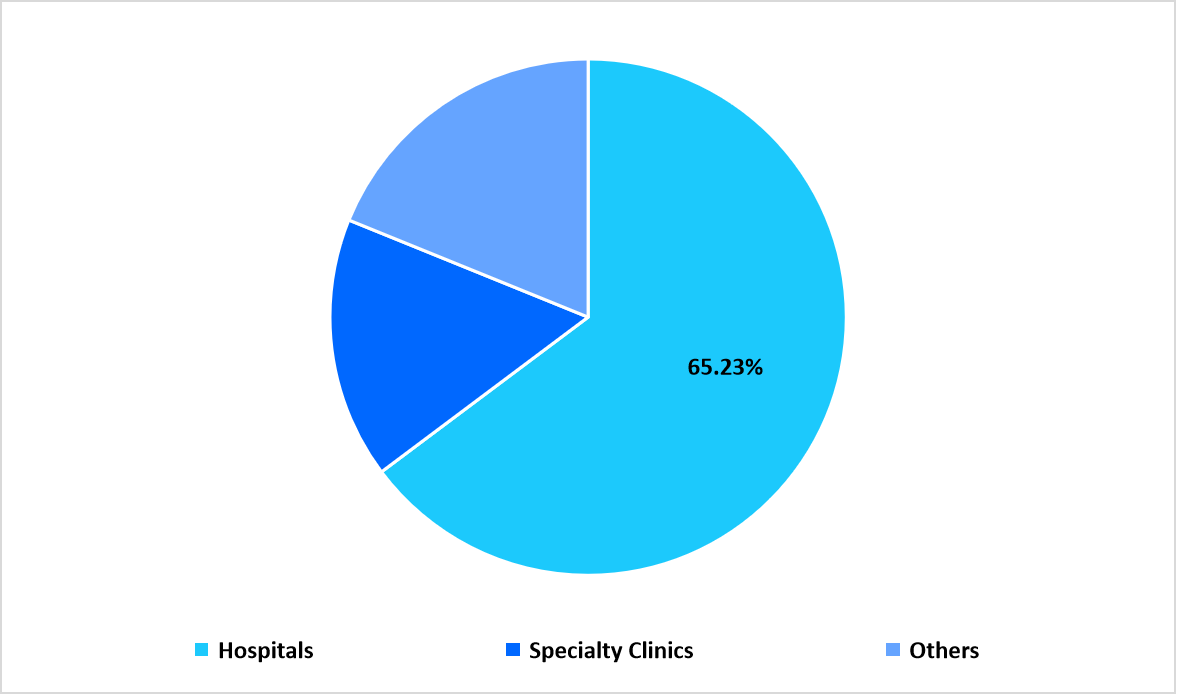
Source: Straits Research
Competitive Landscape
The global interleukin inhibitors market is moderately consolidated, dominated by large multinational pharmaceutical and biotechnology companies with deep biologics portfolios, extensive R&D pipelines, and well-established global commercial networks.
AbbVie Inc.: An Emerging Market Player
AbbVie Inc. leveraged its strong commercial infrastructure and biologics expertise to maintain leading positions in inflammatory and autoimmune therapeutic areas. The company pursued lifecycle extension strategies, global market access efforts, and strategic partnerships to defend and expand its interleukin-targeted franchise while investing in next-generation candidates and manufacturing capacity.
List of Key and Emerging Players in Interleukin Inhibitors Market
- AbbVie Inc.
- Johnson & Johnson
- Novartis AG
- Eli Lilly and Company
- Amgen Inc.
- Regeneron Pharmaceuticals Inc.
- Roche Holding AG
- Pfizer Inc.
- Sanofi
- Bristol Myers Squibb
- AstraZeneca
- GSK plc.
- Merck & Co., Inc.
- Takeda Pharmaceutical Company
- UCB Pharma
- Sun Pharmaceutical Industries Ltd.
- Biogen Inc.
- Teva Pharmaceutical Industries Ltd.
- Boehringer Ingelheim
- Astellas Pharma Inc.
- Others
Strategic Initiatives
- September 2024: Eli Lilly’s lebrikizumab-based drug, EBGLYSS, received FDA approval for use in adults and adolescents aged 12 and older with moderate-to-severe atopic dermatitis that had been inadequately controlled with topical treatments, and it delivered significant symptom relief.
- August 2024: Regeneron Pharmaceuticals Inc. presented 20 abstracts on Dupixent and itepekimab at the ERS Congress, where the company highlighted major advancements in the treatment of respiratory diseases, including COPD, asthma, and chronic rhinosinusitis with nasal polyps.
- June 2024: Johnson & Johnson announced that the Phase 3 GRAVITI study for TREMFYA (guselkumab) had achieved successful results, demonstrating the therapy’s potential as the only IL-23 inhibitor offering both subcutaneous and intravenous induction options.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 36.36 billion |
| Market Size in 2026 | USD 42.54billion |
| Market Size in 2034 | USD 153.09 billion |
| CAGR | 17.36% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Route of Administration, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Interleukin Inhibitors Market Segments
By Type
- IL-1 Inhibitors
- IL-2 Inhibitors
- IL-5 Inhibitors
- IL-6 Inhibitors
- IL-17 Inhibitors
- IL-23 Inhibitors
- Other Types
By Route of Administration
- Subcutaneous (SC)
- Intravenous (IV)
By Application
- Rheumatoid Arthritis
- Psoriasis
- Inflammatory Bowel Disease (IBD)
- Asthma
- Other Applications
By End Use
- Hospitals
- Specialty Clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































