Large Volume Parenteral (LVP) Market Size, Share & Trends Analysis Report By Type (Soft Bag LVP, Plastic Bottle LVP, Glass Bottle LVP), By Volume (100 ML – 250 ML, 250 ML – 500 ML, 500 ML – 1000 ML, 1000 ML – 2000 ML, 2000 ML and More), By Application (Therapeutic Injections, Fluid Balance Injections, Nutritious Injections), By End Users (Hospitals, Clinics, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Large Volume Parenteral (lvp) Market Size
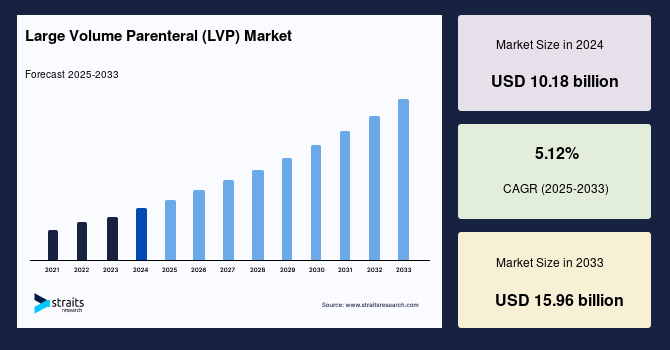
The global large volume parenteral (LVP) market size was valued at USD 10.18 billion in 2024 and is projected to grow from USD 10.70 billion in 2025 to USD 15.96 billion in 2033, exhibiting a CAGR of 5.12% during the forecast period (2025-2033).
The global large volume parenteral (LVP) market is experiencing robust growth, driven by the increasing prevalence of chronic diseases, a rising geriatric population, and the demand for sterile and effective drug delivery systems. LVPs, typically administered intravenously in volumes exceeding 100 mL, are essential in treating conditions like dehydration, electrolyte imbalances, and for delivering medications such as antibiotics and chemotherapy agents. Technological advancements, including improved packaging and drug formulations, are enhancing the safety and efficacy of LVPs. Moreover, the expansion of healthcare infrastructure in emerging economies and the emphasis on patient-centric care are further propelling market growth. However, challenges such as stringent regulatory requirements and the need for cold chain logistics may hinder market expansion.
Latest Market Trend
Technological Advancements in Lvp Delivery Systems
The LVP market is experiencing significant technological innovations to enhance drug delivery efficiency and patient compliance. Advancements include the development of smart infusion pumps and wearable injectors that allow for controlled and precise drug administration. These devices are particularly beneficial for home healthcare settings, reducing hospital stays and associated costs.
- For instance, in April 2024, Baxter International Inc. received FDA 510(k) clearance for its Novum IQ large-volume infusion pump with Dose IQ safety software, featuring a web-based, customisable drug library and dose error reduction system.
Additionally, integrating electronic health records (EHRs) with infusion devices enables real-time monitoring and adjustments, enhancing treatment outcomes. The use of advanced materials in packaging also ensures better sterility and shelf life of LVPs. Such innovations are expected to streamline LVP administration, making treatments more accessible and efficient.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 10.18 Billion |
| Estimated 2025 Value | USD 10.70 Billion |
| Projected 2033 Value | USD 15.96 Billion |
| CAGR (2025-2033) | 5.12% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Baxter International Inc., Fresenius Kabi, Braun Melsungen AG, Pfizer Inc., ICU Medical Inc. |
to learn more about this report Download Free Sample Report
Large Volume Parenteral (lvp) Market Driver
Rising Prevalence of Chronic Diseases
Chronic diseases such as diabetes, cancer, and cardiovascular disorders are on the rise globally, necessitating long-term and often intravenous treatments. LVPs play a crucial role in managing these conditions by injecting essential fluids, electrolytes, and medications into the bloodstream. The increasing burden of chronic illnesses is thus a significant driver for the LVP market, as healthcare providers seek reliable and efficient drug delivery systems to manage patient care.
- According to the World Health Organisation, non-communicable diseases account for approximately 74% of all deaths worldwide, highlighting the critical need for effective treatment modalities like LVPs.
Furthermore, the ageing global population contributes to the rising demand for LVPs, as elderly individuals are more susceptible to chronic conditions requiring intravenous therapies. The integration of smart technologies in LVP delivery systems also supports the management of chronic diseases by enabling precise dosing and monitoring, thereby improving patient outcomes.
Market Restraining Factors
Stringent Regulatory Requirements
The LVP industry is subject to rigorous regulatory standards to ensure patient safety and product efficacy. Manufacturers must comply with stringent drug formulation, packaging, and sterility guidelines. These regulations often require substantial investment in quality control systems and can lead to prolonged approval timelines for new products. Additionally, any lapses in compliance can result in product recalls, legal penalties, and damage to brand reputation. Such regulatory challenges can be particularly burdensome for small and medium-sized enterprises, potentially limiting innovation and market entry. For example, in January 2024, Fresenius Kabi received a Class I rating from the FDA for a recall of the Ivenix medication delivery technology due to issues with the large-volume pump component. This incident underscores the critical importance of adhering to regulatory standards and the potential consequences of non-compliance.
Market Opportunity
Expansion in Emerging Markets
Emerging economies present significant growth opportunities for the LVP market due to increasing healthcare expenditures, improving infrastructure, and a growing patient population. Governments in countries like India and China are investing heavily in healthcare reforms and hospital expansions, creating a conducive environment for LVP adoption. Moreover, the rising awareness about the benefits of early and effective treatment is leading to higher demand for LVPs. Companies that can navigate these markets' regulatory landscapes and establish strong distribution networks are poised to capitalise on these opportunities.
- For example, in May 2024, Bristol Myers Squibb (BMS) launched the ASPIRE strategy to increase the affordability and availability of BMS medicines in low- and middle-income countries (LMICs), aiming to reach more than 200,000 patients by 2033.
The development of environmentally friendly packaging materials and the integration of advanced technologies in LVP delivery systems further enhance the appeal of these products in emerging markets, aligning with global sustainability goals and improving patient care.
Regional Insights
North America continues to lead the global LVP market, with the United States at the forefront. This dominance is attributed to advanced healthcare infrastructure, substantial healthcare expenditure, and the presence of major pharmaceutical companies. The region's high prevalence of chronic diseases, such as diabetes and cancer, necessitates the extensive use of LVPs for effective treatment. In 2025, the U.S. Food and Drug Administration (FDA) streamlined its regulatory process for injectable manufacturing facilities through the Emerging Technology Program (ETP), encouraging the adoption of advanced production lines and sterile packaging. Moreover, companies like Baxter International and Pfizer are expanding their LVP production capacities.
- The U.S. remains the largest market for LVPs globally, driven by a well-developed healthcare system, advanced biopharmaceutical manufacturing capabilities, and a high burden of chronic diseases like cancer, diabetes, and renal disorders. The FDA’s Emerging Technology Program (ETP) has significantly reduced regulatory bottlenecks for sterile injectable facilities, encouraging companies to invest in cutting-edge production technologies. Furthermore, partnerships between hospitals and drug manufacturers promote the use of customised IV bags for patient-specific therapies, a trend gaining traction in large hospital networks.
- Canada’s LVP market is steadily expanding, underpinned by its ageing population, high incidence of chronic diseases, and increasing volumes of inpatient care. Leading global firms such as Fresenius Kabi and B. Braun are strengthening their distribution networks in Canada through partnerships with provincial health systems, enabling real-time supply of sterile, ready-to-administer solutions. These efforts are expected to significantly improve clinical outcomes and streamline intravenous therapy workflows across public hospitals.
Asia-Pacific Large Volume Parenteral Market Trends
The Asia-Pacific region is experiencing rapid growth in the market for LVP, driven by increasing healthcare expenditure, rising awareness about advanced medical treatments, and the expansion of healthcare infrastructure. Countries like China, India, and Japan are at the forefront of this growth. The large patient population and the rising incidence of chronic diseases create substantial demand for LVPs. Government initiatives like India's Ayushman Bharat scheme aim to provide affordable healthcare, thereby increasing the accessibility and utilisation of LVPs. Additionally, the growth of medical tourism in the region contributes to market expansion.
- China is the fastest-growing LVP market globally, thanks to expansive healthcare reforms, rapid hospital network development, and a large ageing population. The “Healthy China 2030” agenda emphasises access to injectable therapies in urban and rural settings. The country’s rising cancer and diabetes burden is fueling demand for LVP-based chemotherapy support and parenteral nutrition. Moreover, digital transformation in hospital supply chains enables better tracking, allocation, and usage of IV fluids across China’s expanding healthcare infrastructure.
- India is rapidly emerging as a global hub for LVP production, leveraging low manufacturing costs and rising domestic demand. The extension of the Production-Linked Incentive (PLI) Scheme for Pharmaceuticals provides fiscal benefits to local firms investing in sterile injectable infrastructure. India’s increasing hospitalisation rates, growing medical tourism sector, and emphasis on emergency preparedness have significantly boosted the need for affordable, high-volume intravenous therapies. Additionally, government-led procurement programs for public hospitals are pushing for ready-to-use LVP solutions to improve treatment efficiency and reduce infection risks.
Europe Large Volume Parenteral Market Trends
Europe holds a significant share in the global LVP industry, with countries like Germany, France, and the United Kingdom leading. The region's well-established healthcare systems, stringent regulatory frameworks, and high adoption of advanced medical technologies support market growth. The increasing burden of chronic diseases and the ageing population drive the demand for LVPs. European governments' focus on improving healthcare quality, patient safety, and investments in healthcare infrastructure further stimulates market development. Collaborations between the public and private sectors in research and innovation also play a crucial role in advancing the LVP market in Europe.
- Germany, a leader in Europe’s biopharmaceutical industry, boasts a mature market for LVP supported by high healthcare spending and a robust manufacturing ecosystem. The Pharma 2030 strategy, backed by the federal government, incentivises domestic production to reduce reliance on imports and improve supply chain resilience. The rising prevalence of chronic conditions such as heart disease and kidney failure has amplified demand for parenteral therapies in both hospitals and home care. Germany's strong regulatory environment and emphasis on quality assurance continue to attract global pharmaceutical investments in the LVP segment.
- France is witnessing robust growth in its LVP industry, driven by national healthcare modernisation programs and increasing surgical admissions. Under the 2024–2027 Health Innovation Plan, the French government provides substantial subsidies to pharmaceutical companies investing in sterile injectables. The country’s focus on enhancing post-operative care, cancer management, and nutritional support drives demand for high-quality parenteral solutions. Additionally, integrating digital infusion monitoring systems in public hospitals is expected to enhance patient safety and optimise LVP usage.
Type Insights
Soft Bag LVPs have emerged as the dominant type in the market due to their numerous advantages over traditional glass or plastic bottles. These include superior flexibility, a reduced risk of contamination, and a more environmentally sustainable footprint. Their collapsible design eliminates the need for air exchange, thus maintaining sterility and preventing microbial ingress. The lightweight nature of soft bags also significantly lowers transportation costs and requires less storage space. Additionally, their compatibility with automated aseptic filling lines improves production scalability and operational efficiency. With hospitals increasingly prioritising single-use systems to curb hospital-acquired infections, soft bags are the preferred choice. Recent innovations, such as multi-chamber soft bags that allow mixing components immediately before administration, further drive their adoption.
Volume Insights
The 500 ML – 1000 ML segment holds a prominent share in the LVP market because it is ideal for various medical applications. This volume suits fluid resuscitation, electrolyte replenishment, parenteral nutrition, and intravenous drug administration. Healthcare professionals often prefer this size because it delivers adequate therapeutic doses while minimising fluid overload risk and waste. It also aligns with global clinical practice standards, making it a convenient option across varied healthcare settings. The growth of this segment is further propelled by the increasing incidence of chronic conditions requiring prolonged intravenous therapy, such as cancer and renal disease. Moreover, many hospitals are standardising this volume in treatment protocols to streamline inventory management and reduce errors.
Application Insights
Therapeutic injections represent the largest and fastest-growing application segment in the market. They are crucial for delivering a wide range of drugs, including antibiotics, analgesics, antipyretics, and chemotherapy agents, directly into the bloodstream for rapid systemic effects. The escalating burden of chronic diseases such as diabetes, cancer, and cardiovascular disorders has significantly increased the reliance on injectable therapies. Furthermore, the global rise in minimally invasive and surgical procedures drives demand for perioperative medication delivery, often administered through LVPs. The evolution of complex biologics and targeted therapies requiring precision dosing has also spurred advancements in LVP-based drug delivery. Pharmaceutical companies increasingly invest in developing LVP-compatible formulations to enhance therapeutic outcomes and patient compliance.
End Users Insights
Hospitals are the dominant end users in the LVP market, consuming the largest share due to their extensive infrastructure, high patient turnover, and complex treatment regimens. They rely on LVPs for multiple clinical uses, including hydration therapy, electrolyte correction, medication infusion, and total parenteral nutrition (TPN). The increasing prevalence of inpatient admissions, particularly among ageing populations and patients with chronic or critical illnesses, further amplifies LVP demand. Hospitals also benefit from in-house compounding and centralised pharmacy systems, which facilitate the safe and efficient use of large-volume infusions. Additionally, the growing adoption of value-based care models encourages hospitals to use LVPs in standardised treatment protocols to improve outcomes and reduce readmission rates. Continuous upgrades in hospital infrastructure in emerging economies are expected to expand the footprint of LVPs in these settings.
Company Market Share
The global large volume parenteral (LVP) market is fragmented, with multinational giants and regional manufacturers competing on innovation, pricing, and distribution. Leading players follow a strategy of regional expansion, capacity building, and product innovation (especially ready-to-administer formulations). Mergers, acquisitions, and government collaboration are key strategic patterns. The market is marked by consistent investment in sterile processing technologies and partnerships with hospitals and diagnostic centres to improve therapy access.
Fresenius Kabi: Fresenius Kabi is a global leader in the LVP market, offering a diverse range of IV fluids, nutrition solutions, and injectable drugs. The company has a strong footprint in Europe and North America and continues expanding in Asia-Pacific. Its strategy emphasises R&D investment, local manufacturing, and partnerships with public health systems. In January 2025, it completed the acquisition of a sterile injectable plant in Malaysia, boosting its supply chain resilience in the Asia-Pacific region.
Latest News:
- In March 2025, Fresenius Kabi announced the launch of a new line of ready-to-use oncology LVP infusions across European hospitals, addressing the rising demand for time-saving, contamination-free solutions.
List of Key and Emerging Players in Large Volume Parenteral (LVP) Market
- Baxter International Inc.
- Fresenius Kabi
- Braun Melsungen AG
- Pfizer Inc.
- ICU Medical Inc.
- Terumo Corporation
- Otsuka Pharmaceutical
- Vifor Pharma
- JW Life Science
- Kelun Pharmaceutical
- Aculife Healthcare
- Amanta Healthcare
- Grifols S.A.
- Sanofi S.A.

to learn more about this report Download Market Share
Recent Developments
- October 2024- Following Hurricane Helene's impact on Baxter's North Carolina plant, B. Braun Medical ramped up production of IV fluids by 20% at its facilities in Irvine, California, and Daytona Beach, Florida. This increase in production aims to address the nationwide IV fluid shortage and ensure the continuous supply of critical medical solutions to healthcare providers.
- October 2024- Fresenius Medical Care announced plans to increase production of IV fluids and peritoneal dialysis (PD) products to alleviate shortages caused by disruptions in the supply chain. The company is maximising production capacity at its international sites to help add supply amid the industrywide shortage of PD products and IV fluids.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 10.18 Billion |
| Market Size in 2025 | USD 10.70 Billion |
| Market Size in 2033 | USD 15.96 Billion |
| CAGR | 5.12% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Volume, By Application, By End Users |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Large Volume Parenteral (LVP) Market Segments
By Type
- Soft Bag LVP
- Plastic Bottle LVP
- Glass Bottle LVP
By Volume
- 100 ML – 250 ML
- 250 ML – 500 ML
- 500 ML – 1000 ML
- 1000 ML – 2000 ML
- 2000 ML and More
By Application
- Therapeutic Injections
- Fluid Balance Injections
- Nutritious Injections
By End Users
- Hospitals
- Clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































